Synthesis of N-H Bearing Imidazolidinones and Dihydroimidazolones Using Aza-Heck Cyclizations
- PMID: 30125443
- PMCID: PMC6141047
- DOI: 10.1002/anie.201806295
Synthesis of N-H Bearing Imidazolidinones and Dihydroimidazolones Using Aza-Heck Cyclizations
Abstract
The synthesis of unsaturated, unprotected imidazolidinones via an aza-Heck reaction is described. This palladium-catalyzed process allows for the cyclization of N-phenoxy ureas onto pendant alkenes. The reaction has broad functional group tolerance, can be applied to complex ring topologies, and can be used to directly prepare mono- and bis-unprotected imidazolidinones. By addition of Bu4 NI, dihydroimidazolones can be accessed from the same starting materials. Improved conditions for preparing unsaturated, unprotected lactams are also reported.
Keywords: aza-Heck reaction; catalysis; dihydroimidazolones; imidazolidinones; palladium.
© 2018 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures
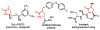

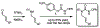

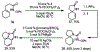
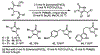


References
-
- Wright WB, Brabander HJ, Hardy RA, Osterberg AC, J. Med. Chem 1966, 9, 852–857 - PubMed
- Lien EJ, Hussain M, Golden MP, J. Med. Chem 1970, 13, 623–626 - PubMed
- Li C-D, Mella SL, Sartorelli AC, J. Med. Chem 1981, 24, 1089–1092 - PubMed
- Salituro FG, Baker CT, Court JJ, Deininger DD, Kim EE, Li B, Novak PM, Rao BG, Pazhanisamy S, Porter MD, Schairer WC, Tung RD, Biorg. Med. Chem. Lett 1998, 8, 3637–3642 - PubMed
- Moreau F, Florentin D, Marquet A, Tetrahedron 2000, 56, 285–293
- Meissner RS, Perkins JJ, Duong LT, Hartman GD, Hoffman WF, Huff JR, Ihle NC, Leu C-T, Nagy RM, Naylor-Olsen A, Rodan GA, Rodan SB, Whitman DB, Wesolowski GA, Duggan ME, Biorg. Med. Chem. Lett 2002, 12, 25–29 - PubMed
- Kazmierski WM, Furfine E, Gray-Nunez Y, Spaltenstein A, Wright L, Biorg. Med. Chem. Lett 2004, 14, 5685–5687 - PubMed
- Shue H-J, Chen X, Shih N-Y, Blythin DJ, Paliwal S, Lin L, Gu D, Schwerdt JH, Shah S, Reichard GA, Piwinski JJ, Duffy RA, Lachowicz JE, Coffin VL, Liu F, Nomeir AA, Morgan CA, Varty GB, Biorg. Med. Chem. Lett 2005, 15, 3896–3899 - PubMed
- Kwak S-H, Bang S-C, Seo H-H, Shin H-R, Lee K-C, Hoang LTA, Jung S-H, Arch. Pharmacal Res 2006, 29, 721–727 - PubMed
- Pirard B, Matter H, J. Med. Chem 2006, 49, 51–69 - PubMed
- Shue H-J, Chen X, Schwerdt JH, Paliwal S, Blythin DJ, Lin L, Gu D, Wang C, Reichard GA, Wang H, Piwinski JJ, Duffy RA, Lachowicz JE, Coffin VL, Nomeir AA, Morgan CA, Varty GB, Shih N-Y, Biorg. Med. Chem. Lett 2006, 16, 1065–1069 - PubMed
- Li JJ, Sutton JC, Nirschl A, Zou Y, Wang H, Sun C, Pi Z, Johnson R, Krystek SR, Seethala R, Golla R, Sleph PG, Beehler BC, Grover GJ, Fura A, Vyas VP, Li CY, Gougoutas JZ, Galella MA, Zahler R, Ostrowski J, Hamann LG, J. Med. Chem 2007, 50, 3015–3025 - PubMed
- Sun S, Zhang Z, Kodumuru V, Pokrovskaia N, Fonarev J, Jia Q, Leung P-Y, Tran J, Ratkay LG, McLaren DG, Radomski C, Chowdhury S, Fu J, Hubbard B, Winther MD, Dales NA, Biorg. Med. Chem. Lett 2014, 24, 520–525 - PubMed
- Forte B, Malgesini B, Piutti C, Quartieri F, Scolaro A, Papeo G, Mar. Drugs 2009, 7, 705–753 - PMC - PubMed
- Ma Y, De S, Chen C, Tetrahedron 2015, 71, 1145–1173. - PMC - PubMed
-
- Robinson RP, Laird ER, Donahue KM, Lopresti-Morrow LL, Mitchell PG, Reese MR, Reeves LM, Rouch AI, Stam EJ, Yocum SA, Biorg. Med. Chem. Lett 2001, 11, 1211–1213 - PubMed
- Reichard GA, Stengone C, Paliwal S, Mergelsberg I, Majmundar S, Wang C, Tiberi R, McPhail AT, Piwinski JJ, Shih N-Y, Org. Lett 2003, 5, 4249–4251 - PubMed
- Robinson DM, Curran MP, Lyseng-Williamson KA, Drugs 2007, 67, 1359–1378. - PubMed
-
- Viso A, Fernández de la Pradilla R, García A, Flores A, Chem. Rev 2005, 105, 3167–3196. - PubMed
-
- Tamaru Y, Hojo M, Higashimura H, Yoshida Z, J. Am. Chem. Soc 1988, 110, 3994–4002
- Harayama H, Okuno H, Takahashi Y, Kimura M, Fugami K, Tanaka S, Tamaru Y, Tetrahedron Lett 1996, 37, 7287–7290
- Rao W-H, Yin X-S, Shi B-F, Org. Lett 2015, 17, 3758–3761 - PubMed
- Shen K, Wang Q, Chem. Sci 2015, 6, 4279–4283 - PMC - PubMed
- Xiong P, Xu F, Qian X-Y, Yohannes Y, Song J, Lu X, Xu H-C, Chem. Eur. J 2016, 22, 4379–4383 - PubMed
- Shen K, Wang Q, J. Am. Chem. Soc 2017, 139, 13110–13116. - PMC - PubMed
-
- Fritz JA, Nakhla JS, Wolfe JP, Org. Lett 2006, 8, 2531–2534 - PMC - PubMed
- Wolfe JP, Eur. J. Org. Chem 2007, 2007, 571–582 - PMC - PubMed
- Fritz JA, Wolfe JP, Tetrahedron 2008, 64, 6838–6852 - PMC - PubMed
- Hopkins BA, Wolfe JP, Angew. Chem. Int. Ed 2012, 51, 9886–9890 - PMC - PubMed
- Babij NR, Wolfe JP, Angew. Chem. Int. Ed 2013, 52, 9247–9250 - PMC - PubMed
- Shen K, Wang Q, Chem. Sci 2017, 8, 8265–8270 - PMC - PubMed
- Jia J, Ho YA, Bülow RF, Rueping M, Chem. Eur. J 2018, Early View, 10.1002/chem.201802907. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

