Ethanol targets nucleoredoxin/dishevelled interactions and stimulates phosphatidylinositol 4-phosphate production in vivo and in vitro
- PMID: 30125555
- PMCID: PMC6297114
- DOI: 10.1016/j.bcp.2018.08.021
Ethanol targets nucleoredoxin/dishevelled interactions and stimulates phosphatidylinositol 4-phosphate production in vivo and in vitro
Abstract
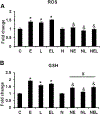
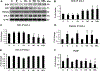
Nucleoredoxin (NXN) is a redox-regulating protein potentially targeted by reactive oxygen species (ROS). It regulates molecular pathways that participate in several key cellular processes. However, the role of NXN in the alcohol liver disease (ALD) redox regulation has not been fully understood. Here, we investigated the effects of ethanol and ethanol plus lipopolysaccharide, a two-hit liver injury model (Ethanol/LPS), on NXN/dishevelled (DVL) interaction and on DVL-dependent phosphoinositides production both in mouse liver and in a co-culture system consisting of human hepatic stellate cells (HSC) and ethanol metabolizing-VL17A human hepatocyte cells. Ethanol and two-hit model increased Nxn protein and mRNA expression, and 4-hydroxynonenal adducts. Two-hit model promoted Nxn nuclear translocation and Dvl/Phosphatidylinositol 4-kinase type-IIα (Pi4k2a) interaction ratio but surprisingly decreased Dvl protein and mRNA levels and reverted ethanol-induced Nxn/Dvl and Dvl/frizzled (Fzd) interaction ratios. Ethanol resulted in a significant increase of Dvl protein and mRNA expression, and decreased Nxn/Dvl interaction ratio but promoted the interaction of Dvl with Fzd and Pi4k2a; formation of this complex induced phosphatidylinositol 4-phosphate [PI(4)P] production. Ethanol and LPS treatments provoked similar alterations on NXN/DVL interaction and its downstream effect in HSC/VL17A co-culture system. Interestingly, ROS and glutathione levels as well as most of ethanol-induced alterations were modified by NXN overexpression in the co-culture system. In conclusion, two-hit model of ethanol exposure disrupts NXN/DVL homeostatic status to allow DVL/FZD/PI4K2A complex formation and stimulates PI(4)P production. These results provide a new mechanism showing that NXN also participates in the regulation of phosphoinositides production that is altered by ethanol during alcoholic liver disease progression.
Keywords: Alcoholic liver disease; Nucleoredoxin; Oxidative stress; Phosphoinositide; Redox regulation.
Copyright © 2018 Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest
Authors have no conflict of interest.
Figures


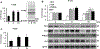



References
-
- Sid B, Verrax J, Calderon PB, Role of oxidative stress in the pathogenesis of alcohol-induced liver disease, Free radical research 47(11) 894–904. - PubMed
-
- Koteish A, Yang S, Lin H, Huang X, Diehl AM, Chronic ethanol exposure potentiates lipopolysaccharide liver injury despite inhibiting Jun N-terminal kinase and caspase 3 activation, The Journal of biological chemistry 277(15) (2002) 13037–44. - PubMed
-
- Gustot T, Lemmers A, Moreno C, Nagy N, Quertinmont E, Nicaise C, Franchimont D, Louis H, Deviere J, Le Moine O, Differential liver sensitization to toll-like receptor pathways in mice with alcoholic fatty liver, Hepatology (Baltimore, Md 43(5) (2006) 989–1000. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

