Continuation of subcutaneous or intramuscular injectable contraception when administered by facility-based and community health workers: findings from a prospective cohort study in Burkina Faso and Uganda
- PMID: 30125558
- PMCID: PMC6197835
- DOI: 10.1016/j.contraception.2018.08.007
Continuation of subcutaneous or intramuscular injectable contraception when administered by facility-based and community health workers: findings from a prospective cohort study in Burkina Faso and Uganda
Abstract
Objective: The aim of this study was to examine continuation of subcutaneous and intramuscular depot medroxyprogesterone acetate (DMPA-SC and DMPA-IM) when administered by facility-based health workers in Burkina Faso and Village Health Teams (VHTs) in Uganda.
Study design: Participants were family planning clients of health centers (Burkina Faso) or VHTs (Uganda) who had decided to initiate injectable use. Women selected DMPA-SC or DMPA-IM and study staff followed them for up to four injections (providing 12 months of pregnancy protection) to determine contraceptive continuation. Study staff interviewed women at their first injection (baseline), second injection, fourth injection and if they discontinued either product.
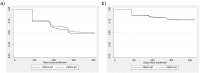
Results: Twelve-month continuation in Burkina Faso was 50% for DMPA-SC and 47.4% for DMPA-IM (p=.41, N=990, 492 DMPA-SC and 498 DMPA-IM). Twelve-month continuation in Uganda was 77.8% for DMPA-SC and 77.4% for DMPA-IM (p=.85, N=1224, 609 DMPA-SC and 615 DMPA-IM). Reasons for discontinuation of DMPA across groups in Burkina Faso included side effects (90/492, 18.3%), being late for injection (68/492, 13.8%) and refusal of spouse (51/492, 10.4%). Reasons for discontinuation in Uganda included being late for injection (65/229, 28.4%), received from non-VHT (50/229, 21.8%) and side effects (34/229, 14.8%). Increased age (adjusted hazard ratio=0.98, p=.01) and partner acceptance of family planning (adjusted hazard ratio=0.48, p<.001) had protective effects against discontinuation in Burkina Faso; we did not find statistically significant variables in Uganda.
Conclusions: There is no difference in 12-month continuation (through four injections) between DMPA-SC and DMPA-IM whether from facility-based health workers in Burkina Faso or VHTs in Uganda. Continuation was higher through community-based distribution in Uganda than health facilities in Burkina Faso.
Implications: The subcutaneous formulation of depot medroxyprogesterone acetate (DMPA-SC) is increasingly available in Family Planning 2020 countries. Use of DMPA-SC does not appear to change continuation relative to traditional intramuscular DMPA. Growing evidence of DMPA-SC's suitability for community-based distribution and self-injection may yield indirect benefits for contraceptive continuation and help reach new users.
Keywords: Community-based distribution; DMPA-IM; DMPA-SC; Injectable contraception; Sayana Press.
Copyright © 2018. Published by Elsevier Inc.
Figures
References
-
- United Nations, Department of Economic and Social Affairs, Population Division . United Nations; New York: 2013. Trends in contraceptive methods use worldwide.
-
- PATH DMPA-SC: expanding contraceptive access and options. 2018. https://www.path.org/articles/dmpa-sc/
-
- Dragoman M.V., Gaffield M.E. The safety of subcutaneously administered depot medroxyprogesterone acetate (104mg/0.65mL): a systematic review. Contraception. 2016;94(3):202–215. - PubMed
-
- Burke H.M., Mueller M.P., Packer C., Perry B., Bufumbo L., Mbengue D. Provider acceptability of Sayana® Press: results from community health workers and clinic-based providers in Uganda and Senegal. Contraception. 2014;89(5):368–373. - PubMed
-
- Burke H.M., Mueller M.P., Perry B., Packer C., Bufumbo L., Mbengue D. Observational study of the acceptability of Sayana® Press among intramuscular DMPA users in Uganda and Senegal. Contraception. 2014;89(5):361–367. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

