Simethicone is retained in endoscopes despite reprocessing: impact of its use on working channel fluid retention and adenosine triphosphate bioluminescence values (with video)
- PMID: 30125574
- PMCID: PMC6754731
- DOI: 10.1016/j.gie.2018.08.012
Simethicone is retained in endoscopes despite reprocessing: impact of its use on working channel fluid retention and adenosine triphosphate bioluminescence values (with video)
Abstract
Background and aims: Studies from our group and others demonstrate residual fluid in 42% to 95% of endoscope working channels despite high-level disinfection and drying. Additionally, persistent simethicone has been reported in endoscope channels despite reprocessing.
Methods: Endoscopy was performed by using water or varied simethicone concentrations (0.5%, 1%, 3%) for flushing. After high-level disinfection/drying, we inspected endoscope working channels for retained fluid by using the SteriCam borescope. Working channel rinsates were evaluated for adenosine triphosphate (ATP) bioluminescence. Fourier transform infrared spectroscopy was performed on fluid droplets gathered from a colonoscope in which low-concentration simethicone was used.
Results: Use of medium/high concentrations of simethicone resulted in a higher mean number of fluid droplets (13.5/17.3 droplets, respectively) and ATP bioluminescence values (20.6/23 relative light units [RLUs], respectively) compared with that of procedures using only water (6.3 droplets/10.9 RLUs; P < .001). Two automated endoscope reprocessing cycles resulted in return of a fluid droplet and ATP bioluminescence values to ranges similar to that of procedures that used only water (P = .56). Low-concentration simethicone did not increase the mean residual fluid or ATP bioluminescence values compared with procedures that used only water (5.8 droplets/15.6 RLUs). Fourier transform infrared analysis revealed simethicone in the endoscope working channel after use of low-concentration simethicone.
Conclusions: Use of medium/high concentrations of simethicone is associated with retention of increased fluid droplets and higher ATP bioluminescence values in endoscope working channels, compared with endoscopes in which water or low concentration simethicone was used. However, simethicone is detectable in endoscopes despite reprocessing, even when it is utilized in low concentrations. Our data suggest that when simethicone is used, it should be used in the lowest concentration possible. Facilities may consider 2 automated endoscope reprocessor cycles for reprocessing of endoscopes when simethicone has been used.
Copyright © 2019 American Society for Gastrointestinal Endoscopy. Published by Elsevier Inc. All rights reserved.
Figures

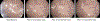

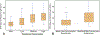
Comment in
-
Innocent bystanders or legitimate culprits? The role of moisture and simethicone in endoscopically transmitted infections.Gastrointest Endosc. 2019 Jan;89(1):133-136. doi: 10.1016/j.gie.2018.09.034. Gastrointest Endosc. 2019. PMID: 30567673 No abstract available.
References
-
- Chang WK, Yeh MK, Hsu HC, et al. Efficacy of simethicone and N-acetylcysteine as premedication in improving visibility during upper endoscopy. J Gastroenterol Hepatol 2014;29:769–74. - PubMed
-
- Catalone BJ. Simethicone: letter to health care practitioner. Center Valley (Pa): Olympus; 2009.
-
- PENTAX Medical Company [instructions for use]. Pentax Video GI Scopes 90i Series 90K Series. Tokyo, Japan: Hoya Corporation; 2014.
-
- Fujinon Films USA ED [reprocessing summary and guide for Fujinon/Fujifilm flexible GI endoscopes]. 2012.
-
- Ofstead CL, Wetzler HP, Johnson EA, et al. Simethicone residue remains inside gastrointestinal endoscopes despite reprocessing. Am J Infect Control 2016;44:1237–40. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

