Discontinuation of intravenous oxytocin in the active phase of induced labour
- PMID: 30125998
- PMCID: PMC6513418
- DOI: 10.1002/14651858.CD012274.pub2
Discontinuation of intravenous oxytocin in the active phase of induced labour
Abstract
Background: In most Western countries, obstetricians and midwives induce labour in about 25% of pregnant women. Oxytocin is an effective drug for this purpose, but associated with serious adverse effects of which uterine tachysystole, fetal distress and the need for immediate delivery are the most common. Various administration regimens such as reduced or pulsatile dosing have been suggested to minimise these. Discontinuation in the active phase of labour, i.e. when contractions are well-established and the cervix is dilated at least 5 cm is another method which may reduce adverse effects.
Objectives: To assess whether birth outcomes can be improved by discontinuation of intravenous (IV) oxytocin, initiated in the latent phase of induced labour, once active phase of labour is established.
Search methods: We searched Cochrane Pregnancy and Childbirth's Trials Register (31 January 2018), Scopus, ClinicalTrials.gov, and the WHO International Clinical Trials Registry Platform (ICTRP) (23 January 2018) together with reference checking, citation searching, and contact with study authors to identify additional studies.
Selection criteria: Randomised controlled trials (RCTs) comparing discontinued IV with continuous IV oxytocin in the active phase of induced labour.No exclusion criteria were applied in terms of parity, maternal age, ethnicity, co-morbidity status, labour setting, gestational age, and prior caesarean delivery.Studies comparing different dosage regimens are outside the scope of this review.
Data collection and analysis: We used standard Cochrane methods.
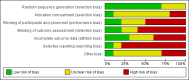
Main results: We found 10 completed RCTs involving 1888 women. One additional trial is ongoing. The included trials were conducted in hospital settings between February 1998 and January 2016, two in Europe (Denmark, and Greece), two in Turkey, and one each in Israel, Iran, USA, Bangladesh, India, and Thailand. Most trials included full-term singleton pregnancies with a fetus in vertex presentation. Some excluded women with cervical priming prior to induction and some excluded women with a history of prior caesarean delivery. When reported, the average age of the women ranged from 22 to 31 years, nulliparity from 45% to 68%, and pre-pregnancy body mass index from 22 to 32.Many of the included trials had design limitations and were judged to be at either high or unclear risk of bias across a number of 'Risk of bias' domains.Four trials included a Consort flow diagram. In three, this gave details of participants delivered before the active phase of labour, and treatment compliance for those who reached that stage. One Consort diagram only provided the latter information. The data in many of the trials without such a flow diagram were implausibly compliant with treatment allocation, suggesting that there had been silent post randomisation exclusions of women delivered before the active phase of labour. We therefore conducted a secondary analysis (not in our protocol) of caesarean section among women who reached the active phase of labour and were therefore eligible for the intervention.Our analysis by 'intention-to-treat' found that, compared with continuation of IV oxytocin stimulation, discontinuation of IV oxytocin may reduce the caesarean delivery rate, risk ratio (RR) 0.69, 95% confidence interval (CI) 0.56 to 0.86, 9 trials, 1784 women, low-level certainty. However, restricting our analysis to women who reached the active phase of labour (using 'reached active phase' as our denominator) suggests there is probably little or no difference between groups (RR 0.92, 95% CI 0.65 to 1.29, 4 trials, 787 women, moderate-certainty evidence).Discontinuation of IV oxytocin probably reduces the risk ofuterine tachysystole combined with abnormal fetal heart rate (FHR) compared with continued IV oxytocin (RR 0.15, 95% CI 0.05 to 0.46, 3 trials, 486 women, moderate-level certainty). We are uncertain about whether or not discontinuation increases the risk of chorioamnionitis (average RR 2.32, 95% CI 0.99 to 5.45, 1 trial, 252 women, very low-level certainty). Discontinuation of IV oxytocin may have little or no impact on the use of analgesia and epidural during labour compared to the use of continued IV oxytocin (RR 1.04 95% CI 0.95 to 1.14, 3 trials, 556 women, low-level certainty). Intrapartum cardiotocography (CTG) abnormalities (suspicious/pathological CTGs) are probably reduced by discontinuing IV oxytocin (RR 0.65, 95% CI 0.51 to 0.83, 7 trials, 1390 women, moderate-level certainty). Compared to continuing IV oxytocin, discontinuing IV oxytocin probably has little or no impact on the incidence of Apgar < 7 at five minutes (RR 0.78, 95% CI 0.27 to 2.21, 4 trials, 893 women, low-level certainty), or and acidotic cord gasses at birth (arterial umbilical pH < 7.10), (RR 1.03, 95% CI 0.50 to 2.13, 4 trials, 873 women, low-level certainty).Many of this review's maternal and infant secondary outcomes (including maternal and neonatal mortality) were not reported in the included trials.
Authors' conclusions: Discontinuing IV oxytocin stimulation after the active phase of labour has been established may reduce caesarean delivery but the evidence for this was low certainty. When restricting our analysis to those trials that separately reported participants who reached the active phase of labour, our results showed there is probably little or no difference between groups. Discontinuing IV oxytocin may reduce uterine tachysystole combined with abnormal FHR.Most of the trials had 'Risk of bias' concerns which means that these results should be interpreted with caution. Our GRADE assessments ranged from very low certainty to moderate certainty. Downgrading decisions were based on study limitations, imprecision and indirectness.Future research could account for all women randomised and, in particular, note those who delivered before the point at which they would be eligible for the intervention (i.e. those who had caesareans in the latent phase), or because labour was so rapid that the infusion could not be stopped in time.Future trials could adopt the outcomes listed in this review including maternal and neonatal mortality, maternal satisfaction, and breastfeeding.
Conflict of interest statement
Sidsel Boie is co‐author on one of the included trials (Bor 2016) and primary investigator of an ongoing double‐blind multicentre randomised controlled trial (RCT) on the topic (NCT02553226). She has/will not be involved in any decisions relating to this review ‐ assessment for inclusion, risk of bias, data extraction and GRADE quality assessments has been/will be carried out by other members of the team who are not directly involved in the trial.
Adeline V Velu: none known.
Julie Glavind: Trygfonden Denmark provided postdoctoral salary support at Aarhus University Hospital from April‐September 2014. She currently receives salary support (as a registrar) from Central Denmark Region. She is an investigator of an ongoing double‐blind multicentre RCT on the topic (NCT02553226). She will not be involved in any decisions relating to this review ‐ assessment for inclusion, risk of bias, data extraction and GRADE quality assessments will be carried out by other members of the team who are not directly involved in the trial.
Ben Willem J Mol and his institution have received payment for consultancy from ObsEva Geneva. Ben has also received payment for review preparation from European Journal of Obstetrics & Gynecology from ESHRE Munich and Prebic Geneva in respect of travel/accommodation/meeting expenses for various non‐commercial scientific meetings
Niels Uldbjerg is an investigator on the ongoing randomised trial, the CONDISOX trial (NCT02553226) that may be considered for inclusion in a future update of this review. He will not be involved in any decisions relating to this review ‐ assessment for inclusion, risk of bias, data extraction and GRADE quality assessments will be carried out by other members of the team who are not directly involved in the trial.
Irene de Graaf: none known.
Pinar Bor is principle investigator on one of the included trials (Bor 2016), and an investigator on a ongoing double‐blind multicentre RCT on the topic (NCT02553226). She has/will not be involved in any decisions relating to this review ‐ assessment for inclusion, risk of bias, data extraction and GRADE quality assessments has been/will be carried out by other members of the team who are not directly involved in the trial.
Jannet JH Bakker is one of the authors of the ongoing randomised trial, the CONDISOX trial (NCT02553226) that may be considered for inclusion in a future update of this review. She will not be involved in any decisions relating to this review ‐ assessment for inclusion, risk of bias, data extraction and GRADE quality assessments will be carried out by other members of the team who are not directly involved in the trial.
JIm Thornton is the chair of the trial steering committee for the ongoing randomised trial, the CONDISOX trial (NCT02553226) that may be considered for inclusion in a future update of this review. He will not be involved in any decisions relating to this review ‐ assessment for inclusion, risk of bias, data extraction and GRADE quality assessments will be carried out by other members of the team who are not directly involved in the trial.
Figures
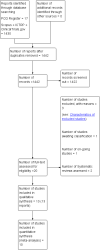















Update of
References
References to studies included in this review
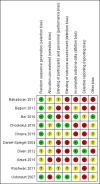
Bahadoran 2011 {published data only}
Begum 2013 {published data only}
-
- Begum LN, Sultana M, Nahar S, Begum R, Barua S. A randomized clinical trial on the need of continuing oxytocin infusion in active phase of induced labour. Chattagram Maa‐O‐Shishu Hospital Medical College Journal 2013;12(2):23‐30.
Bor 2016 {published data only}
-
- Bor P, Ledertoug S, Boie S, Knoblauch NO, Stornes I. Continuation versus discontinuation of oxytocin infusion during the active phase of labour: a randomised controlled trial. BJOG: an international journal of obstetrics and gynaecology 2016;123(1):129‐35. - PubMed
Chookijkul 2016 {published data only}
-
- Chookijkul L, Prommas S, Pariyawateekul P, Orungrote N, Smanchat B, Suwannarurk K. Cesarean section rate in oxytocin infusion between continuous until delivery and discontinuation at active phase of labor: A randomized controlled study. Thai Journal of Obstetrics and Gynaecology 2016;24:73‐80.
-
- Chookijkul L, TCTR20150503001. Cesarean section rate in oxytocin infusion between continuous until delivery and discontinuation at active phase of labor: a randomized controlled study. http://www.clinicaltrials.in.th/index.php?tp=regtrials&menu=trialsea... (first received 3 May 2015).
Chopra 2015 {published data only}
-
- Sengupta SK, Jain V, Chopra S, Kumar P. Oxytocin discontinuation in active phase: the effects. BJOG: an international journal of obstetrics and gynaecology 2014;121(Suppl 2):88.
Daniel‐Spiegel 2004 {published data only}
-
- Daniel‐Spiegel E, Weiner Z, Ben‐Shlomo I, Shalev E. For how long should oxytocin be continued during induction of labour?. BJOG: an International Journal of Obstetrics and Gynaecology 2004;111(4):331‐4. - PubMed
Diven 2012 {published data only}
-
- Diven L, Gogle J, Eid S, Smulian J, Quinones J. Induction of labor with oxytocin: should oxytocin be held?. American Journal of Obstetrics and Gynecology 2012;206(Suppl 1):S144. - PubMed
-
- Diven LC, Rochon ML, Gogle J, Eid S, Smulian JC, Quinones JN. Oxytocin discontinuation during active labor in women who undergo labor induction. American Journal of Obstetrics and Gynecology 2012;207(6):471.e1‐471e8. - PubMed
Ozturk 2015 {published data only}
-
- Ozturk FH, Yilmaz SS, Yalvac S, Kandemir O. Effect of oxytocin discontinuation during the active phase of labor. Journal of Maternal‐Fetal & Neonatal Medicine 2015;28(2):196‐8. - PubMed
Rashwan 2011 {published data only}
-
- Rashwan ASSA, Gaafar HM, Mohamed AMM. Comparative study between continuous use of oxytocin infusion throughout the active phase of labor versus its discontinuation and its effect on the course of labor. Medical Journal of Cairo University 2011;79(2):121‐5.
Ustunyurt 2007 {published data only}
-
- Ustunyurt E, Ugur M, Ustunyurt BO, Iskender TC, Ozkan O, Mollamahmutoglu L. Prospective randomized study of oxytocin discontinuation after the active stage of labor is established. Journal of Obstetrics and Gynaecology Research 2007;33(6):799‐803. - PubMed
References to studies excluded from this review
D'Souza 1986 {published data only}
-
- D'Souza SW, Lieberman B, Cadman J, Richards B. Oxytocin induction of labour: hyponatraemia and neonatal jaundice. European Journal Obstetrics & Gynecology and Reproductive Biology 1986;22:309‐17. - PubMed
Girard 2009 {published data only}
-
- Girard B, Vardon D, Creveuil C, Herlicoviez M, Dreyfus M. Discontinuation of oxytocin in the active phase of labor. Acta Obstetricia et Gynecologica Scandinavica 2009;88(2):172‐7. - PubMed
Pacheco 2006 {published data only}
-
- Pacheco LD, Rosen MP, Gei AF, Saade GR, Hankins GD. Management of uterine hyperstimulation with concomitant use of oxytocin and terbutaline. American Journal of Perinatology 2006;23(6):377‐80. - PubMed
References to studies awaiting assessment
Abdelhamid 2010 {published data only}
-
- Abdelhamid EE. For how long should oxytocin be continued during induction of labour?. Jamahiriya Medical Journal 2010;10(4):251‐3. [CENTRAL: CN‐01016774]
References to ongoing studies
NCT02553226 {published data only}
-
- NCT02553226. Continued versus discontinued oxytocin stimulation of labour in a double‐blind randomised controlled trial. clinicaltrials.gov/ct2/show/NCT02553226 (first received 17 September 2015).
Additional references
ACOG 2009
-
- Anon. ACOG Practice Bulletin No. 107: Induction of labor. Obstetrics and Gynecology 2009;114(2 Pt 1):386‐97. [PUBMED: 19623003] - PubMed
ACOG 2014
Bakker 2007
-
- Bakker PC, Kurver PH, Kuik DJ, Geijn HP. Elevated uterine activity increases the risk of fetal acidosis at birth. American Journal of Obstetrics and Gynecology 2007;196(4):313‐6. - PubMed
Budden 2014
Cahill 2008
-
- Cahill AG, Waterman BM, Stamilio DM, Odibo AO, Allsworth JE, Evanoff B, et al. Higher maximum doses of oxytocin are associated with an unacceptably high risk for uterine rupture in patients attempting vaginal birth after cesarean delivery. American Journal of Obstetrics and Gynecology 2008;199(1):32e1‐5. [DOI: 10.1016/j.ajog.2008.03.001] - DOI - PubMed
Clark 2008
-
- Clark SL, Belfort MA, Dildy GA, Meyers JA. Reducing obstetric litigation through alterations in practice patterns. Obstetrics and Gynecology 2008;112(6):1279‐83. [PUBMED: 19037036] - PubMed
Dahlen 2013
Dansereau 1999
-
- Dansereau J, Joshi AK, Helewa ME, Doran AT, Lange ER, Luther IR. Double‐blind comparison of carbetocin versus oxytocin in prevention of uterine atony after caesarean section. American Journal of Obstetrics and Gynecology 1999;180(3 Pt 1):670‐6. - PubMed
Dencker 2010
Fernandez 2012
-
- Fernandez O, Marin GM, Malalana MA, Fernandez‐Cañadas MA, Lopez SF, Costareli V. Newborn feeding behaviour depressed by intrapartum oxytocin: a pilot study. Acta Paediatrica 2012;101(7):749‐54. - PubMed
Grotegut 2011
Hertog 2001
-
- Hertog CE, Groot AN, Dongen PW. History and use of oxytocics. European Journal of Obstetrics, Gynecology, and Reproductive Biology 2001;94(1):8‐12. - PubMed
Higgins 2011
-
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
ISMP List 2014
-
- List of High‐Alert Medications in Acute Care Settings. Institute for Safe Medication Practices (ISMP) (http://www.ismp.org/tools/highalertmedications.pdf) 2014.
Malek 1996
-
- Malek A, Blann E, Mattison DR. Human placental transport of oxytocin. Journal of Maternal and Fetal Medicine 1996;5(5):245‐55. [PUBMED: 8930795] - PubMed
NICE 2017
-
- National Collaborating Centre for Women's and Children's Health (UK) et al. Intrapartum care: care of healthy women and their babies during childbirth. National Institute for Health and Care Excellence December 2014 (updated February 2017). [PUBMED: 25950072] - PubMed
Oláh 2015
-
- Oláh KSJ, Steer PJ. The use and abuse of oxytocin. Obstetrician & Gynaecologist 2015;17(4):265‐71. [DOI: 10.1111/tog.12222] - DOI
Oscarsson 2006
-
- Oscarsson ME, Amer‐Wahlin I, Rydhstroem H, Källén K. Outcome in obstetric care related to oxytocin use. A population‐based study. Acta Obstetricia et Gynecologica Scandinavica 2006;85(9):1094‐8. - PubMed
Phaneuf 2000
-
- Phaneuf S, Rodriguez Liñares B, TambyRaja RL, MacKenzie IZ, López Bernal A. Loss of myometrial oxytocin receptors during oxytocin‐induced and oxytocin‐augmented labour. Journal of Reproduction and Fertile 2000;120(1):91‐7. - PubMed
RevMan 2014 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Saccone 2017
Selin 2009
-
- Selin L, Almstrom E, Wallin G, Berg M. Use and abuse of oxytocin for augmentation of labor. Acta Obstetricia et Gynecologica Scandinavica 2009;88(12):1352‐7. - PubMed
Simpsons 2009
-
- Simpson KR, Knox GE. Oxytocin as a high‐alert medication: implications for perinatal patient safety. MCN. American Journal of Maternal Child Nursing 2009;34(1):8‐15. - PubMed
Svare 2014
-
- Svare JA, Hansen BB, Lose G. Risk factors for urinary incontinence 1 year after the first vaginal delivery in a cohort of primiparous Danish women. International Urogynecology Journal 2014;25(1):47‐51. - PubMed
References to other published versions of this review
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

