Neutrophil-to-lymphocyte ratio after four weeks of nivolumab administration as a predictive marker in patients with pretreated non-small-cell lung cancer
- PMID: 30126063
- PMCID: PMC6166075
- DOI: 10.1111/1759-7714.12838
Neutrophil-to-lymphocyte ratio after four weeks of nivolumab administration as a predictive marker in patients with pretreated non-small-cell lung cancer
Abstract
Background: Although phase III trials have shown improved overall and progression-free survival (PFS) using nivolumab compared to docetaxel in patients with non-small-cell lung cancer, the progressive disease ratio of nivolumab is higher than docetaxel. Furthermore, nonconventional response patterns of nivolumab make it difficult to determine the time point for nivolumab discontinuation. Therefore, a method to detect non-responders to nivolumab at an early time point is crucial. This retrospective study was conducted to identify immunological and nutritional markers, including neutrophil-to-lymphocyte ratios (NLR), which would predict the efficacy of nivolumab treatment. Because the expression of these markers fluctuates dramatically during treatment, repeat evaluation was performed.
Methods: We retrospectively investigated 30 patients with non-small-cell lung cancer who were treated with nivolumab. The stratified data of each marker obtained during four weeks after nivolumab treatment were evaluated by Cox proportional hazards regression to verify the differences in PFS.
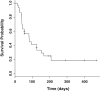
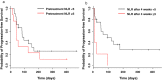
Results: One and four patients experienced progressive disease within two and four weeks, respectively. Therefore, 29 and 26 patients were analyzed two and four weeks after nivolumab administration, respectively. The results showed that the NLR after four weeks could predict PFS. The median PFS in 21 patients with NLR < 5 after four weeks of nivolumab administration was 95 days (95% confidence interval [CI] 50-NA), while the mPFS in five patients with NLR ≥ 5 was 10 days (95% CI 6-NA). NLR ≥ 5 showed a hazard ratio of 5.995 (95% CI 1.225-29.35).
Conclusion: Clarifying NLR four weeks after nivolumab administration may be useful to predict outcomes in nivolumab-treated patients.
Keywords: Immunological and nutritional status; neutrophil-to-lymphocyte ratio (NLR); nivolumab; non-small-cell lung cancer; predictive marker.
© 2018 The Authors. Thoracic Cancer published by China Lung Oncology Group and John Wiley & Sons Australia, Ltd.
Figures
References
-
- Chen DS, Mellman I. Oncology meets immunology: The cancer‐immunity cycle. Immunity 2013; 39: 1–10. - PubMed
-
- Xia B, Herbst RS. Immune checkpoint therapy for non‐small‐cell lung cancer: An update. Immunotherapy 2016; 8: 279–98. - PubMed
-
- Nishimura H, Minato N, Nakano T, Honjo T. Immunological studies on PD‐1 deficient mice: Implication of PD‐1 as a negative regulator for B cell responses. Int Immunol 1998; 10: 1563–72. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

