Magnetoencephalography: Clinical and Research Practices
- PMID: 30126121
- PMCID: PMC6120049
- DOI: 10.3390/brainsci8080157
Magnetoencephalography: Clinical and Research Practices
Abstract
Magnetoencephalography (MEG) is a neurophysiological technique that detects the magnetic fields associated with brain activity. Synthetic aperture magnetometry (SAM), a MEG magnetic source imaging technique, can be used to construct both detailed maps of global brain activity as well as virtual electrode signals, which provide information that is similar to invasive electrode recordings. This innovative approach has demonstrated utility in both clinical and research settings. For individuals with epilepsy, MEG provides valuable, nonredundant information. MEG accurately localizes the irritative zone associated with interictal spikes, often detecting epileptiform activity other methods cannot, and may give localizing information when other methods fail. These capabilities potentially greatly increase the population eligible for epilepsy surgery and improve planning for those undergoing surgery. MEG methods can be readily adapted to research settings, allowing noninvasive assessment of whole brain neurophysiological activity, with a theoretical spatial range down to submillimeter voxels, and in both humans and nonhuman primates. The combination of clinical and research activities with MEG offers a unique opportunity to advance translational research from bench to bedside and back.
Keywords: epilepsy; magnetic source imaging; magnetoencephalography; synthetic aperture magnetometry.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


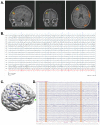

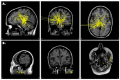
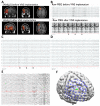

References
-
- Baillet S., Mosher J.C., Leahy R.M. Electromagnetic brain mapping. IEEE Signal Process. Mag. 2001;18:14–30. doi: 10.1109/79.962275. - DOI
-
- Caton R. The Electric Currents of the Brain. BMJ. 1875;2:278. doi: 10.1080/00029238.1970.11080764. - DOI
-
- Hillebrand A., Barnes G.R., Hubert P. International Review of Neurobiology. Volume 68. Academic Press; New York, NY, USA: 2005. Beamformer Analysis of MEG Data; pp. 149–171. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

