The Pathophysiology of Post-Traumatic Glioma
- PMID: 30126222
- PMCID: PMC6121393
- DOI: 10.3390/ijms19082445
The Pathophysiology of Post-Traumatic Glioma
Abstract
Malignant glioma is a brain tumor with a very high mortality rate resulting from the specific morphology of its infiltrative growth and poor early detection rates. The causes of one of its very specific types, i.e., post-traumatic glioma, have been discussed for many years, with some studies providing evidence for mechanisms where the reaction to an injury may in some cases lead to the onset of carcinogenesis in the brain. In this review of the available literature, we discuss the consequences of breaking the blood⁻brain barrier and consequences of the influx of immune-system cells to the site of injury. We also analyze the influence of inflammatory mediators on the expression of genes controlling the process of apoptosis and the effect of chemical mutagenic factors on glial cells in the brain. We present the results of experimental studies indicating a relationship between injury and glioma development. However, epidemiological studies on post-traumatic glioma, of which only a few confirm the conclusions of experimental research, indicate that any potential relationship between injury and glioma, if any, is indirect.
Keywords: IL-6; STAT3; blood–brain barrier; brain; injury; pathophysiology; post-traumatic glioma.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
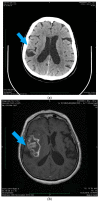

References
-
- Tyagi V., Theobald J., Barger J., Bustoros M., Bayin N.S., Modrek A.S., Kader M., Anderer E.G., Donahue B., Fatterpekar G., et al. Traumatic brain injury and subsequent glioblastoma development: Review of the literature and case reports. Surg. Neurol. Int. 2016;26:77–78. doi: 10.4103/2152-7806.189296. - DOI - PMC - PubMed
-
- Ostrom Q.T., Gittleman H., Liao P., Rouse C., Chen Y., Dowling J., Wolinsky Y., Kruchko C., Barnholtz-Sloan J. CBTRUS statistical report: Primary brain and central nervous system tumors diagnosed in the United States in 2007–2011. Neuro Oncol. 2014;16:61–63. doi: 10.1093/neuonc/nou223. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

