Trabectedin arrests a doxorubicin-resistant PDGFRA-activated liposarcoma patient-derived orthotopic xenograft (PDOX) nude mouse model
- PMID: 30126369
- PMCID: PMC6102848
- DOI: 10.1186/s12885-018-4703-0
Trabectedin arrests a doxorubicin-resistant PDGFRA-activated liposarcoma patient-derived orthotopic xenograft (PDOX) nude mouse model
Abstract
Background: Pleomorphic liposarcoma (PLPS) is a rare, heterogeneous and an aggressive variant of liposarcoma. Therefore, individualized therapy is urgently needed. Our recent reports suggest that trabectedin (TRAB) is effective against several patient-derived orthotopic xenograft (PDOX) mouse models. Here, we compared the efficacy of first-line therapy, doxorubicin (DOX), and TRAB in a platelet-derived growth factor receptor-α (PDGFRA)-amplified PLPS.
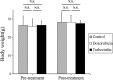
Methods: We used a fresh sample of PLPS tumor derived from a 68-year-old male patient diagnosed with a recurrent PLPS. Subcutaneous implantation of tumor tissue was performed in a nude mouse. After three weeks of implantation, tumor tissues were isolated and cut into small pieces. To match the patient a PDGFRA-amplified PLPS PDOX was created in the biceps femoris of nude mice. Mice were randomized into three groups: Group 1 (G1), control (untreated); Group 2 (G2), DOX-treated; Group 3 (G3), TRAB-treated. Measurement was done twice a week for tumor width, length, and mouse body weight.
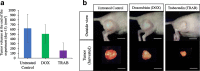
Results: The PLPS PDOX showed resistance towards DOX. However, TRAB could arrest the PLPS (p < 0.05 compared to control; p < 0.05 compared to DOX) without any significant changes in body-weight.
Conclusions: The data presented here suggest that for the individual patient the PLPS PDOX model could specifically distinguish both effective and ineffective drugs. This is especially crucial for PLPS because effective first-line therapy is harder to establish if it is not individualized.
Keywords: Liposarcoma; PDGFRA amplification; PDOX; Patient-derived orthotopic xenograft; Precision medicine; Trabectedin.
Conflict of interest statement
Ethics approval and consent to participate
Written informed consent was obtained from the patient as part of a University of California, Los Angeles (UCLA) Institutional Review Board (IRB#10–001857) approved protocol. Written informed consent was obtained from the patient for publication of the study. All mice investigations were done with an AntiCancer, Inc. Institutional Animal Care and Use Committee (IACUC)-protocol mainly approved for this study and in correspondence with the principals and procedures outlined in the National Institute of Health (NIH) Guide for the Care and Use of Animals under Assurance Number A3873–1.
Consent for publication
Written informed consent was obtained from the patient for publication of the study.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures


Similar articles
-
Eribulin Regresses a Doxorubicin-resistant Dedifferentiated Liposarcoma in a Patient-derived Orthotopic Xenograft Mouse Model.Cancer Genomics Proteomics. 2020 Jul-Aug;17(4):351-358. doi: 10.21873/cgp.20194. Cancer Genomics Proteomics. 2020. PMID: 32576580 Free PMC article.
-
Tumor-targeting Salmonella typhimurium A1-R arrests a doxorubicin-resistant PDGFRA-amplified patient-derived orthotopic xenograft mouse model of pleomorphic liposarcoma.J Cell Biochem. 2018 Sep;119(9):7827-7833. doi: 10.1002/jcb.27183. Epub 2018 Jun 22. J Cell Biochem. 2018. PMID: 29932244 Free PMC article.
-
Doxorubicin-resistant pleomorphic liposarcoma with PDGFRA gene amplification is targeted and regressed by pazopanib in a patient-derived orthotopic xenograft mouse model.Tissue Cell. 2018 Aug;53:30-36. doi: 10.1016/j.tice.2018.05.010. Epub 2018 May 22. Tissue Cell. 2018. PMID: 30060824
-
Complete response of a recurrent-metastatic liposarcoma with dedifferentiated histological features following the administration of trabectedin and review of literature.J Cancer Res Ther. 2015 Oct-Dec;11(4):974-6. doi: 10.4103/0973-1482.158032. J Cancer Res Ther. 2015. PMID: 26881560 Review.
-
Trabectedin combined with liposomal doxorubicin in women with relapsed ovarian cancer.Expert Rev Anticancer Ther. 2010 Jun;10(6):795-805. doi: 10.1586/era.10.59. Expert Rev Anticancer Ther. 2010. PMID: 20553205 Review.
Cited by
-
PDX models for functional precision oncology and discovery science.Nat Rev Cancer. 2025 Mar;25(3):153-166. doi: 10.1038/s41568-024-00779-3. Epub 2024 Dec 16. Nat Rev Cancer. 2025. PMID: 39681638 Free PMC article. Review.
-
Eribulin Regresses a Doxorubicin-resistant Dedifferentiated Liposarcoma in a Patient-derived Orthotopic Xenograft Mouse Model.Cancer Genomics Proteomics. 2020 Jul-Aug;17(4):351-358. doi: 10.21873/cgp.20194. Cancer Genomics Proteomics. 2020. PMID: 32576580 Free PMC article.
-
Tumor-targeting Salmonella typhimurium A1-R arrests a doxorubicin-resistant PDGFRA-amplified patient-derived orthotopic xenograft mouse model of pleomorphic liposarcoma.J Cell Biochem. 2018 Sep;119(9):7827-7833. doi: 10.1002/jcb.27183. Epub 2018 Jun 22. J Cell Biochem. 2018. PMID: 29932244 Free PMC article.
-
A perspective on patient-derived orthotopic xenograft (PDOX) mouse models for identification of novel and individualized treatment for sarcoma.Int J Clin Oncol. 2025 Sep;30(9):1707-1721. doi: 10.1007/s10147-025-02801-6. Epub 2025 Jun 10. Int J Clin Oncol. 2025. PMID: 40493147 Review.
References
-
- Manji GA, Schwartz GK. Managing Liposarcomas: cutting through the fat. J Oncol Pract. 2016;12(3):221–227. - PubMed
-
- Agarwal J, Kadakia S, Agaimy A, Ogadzanov A, Khorsandi A, Chai RL. Pleomorphic liposarcoma of the head and neck: presentation of two cases and literature review. Am J Otolaryngol. 2017;38(4):505–507. - PubMed
-
- Ahmed Z, Shah HU, Yaqoob N, et al. Pleomorphic liposarcoma in a ten year old child. J Pak Med Assoc. 2004;54:533–534. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

