A novel and compact review on the role of oxidative stress in female reproduction
- PMID: 30126412
- PMCID: PMC6102891
- DOI: 10.1186/s12958-018-0391-5
A novel and compact review on the role of oxidative stress in female reproduction
Abstract
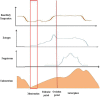
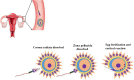
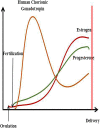
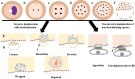
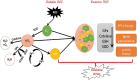
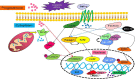
In recent years, the study of oxidative stress (OS) has become increasingly popular. In particular, the role of OS on female fertility is very important and has been focused on closely. The occurrence of OS is due to the excessive production of reactive oxygen species (ROS). ROS are a double-edged sword; they not only play an important role as secondary messengers in many intracellular signaling cascades, but they also exert indispensable effects on pathological processes involving the female genital tract. ROS and antioxidants join in the regulation of reproductive processes in both animals and humans. Imbalances between pro-oxidants and antioxidants could lead to a number of female reproductive diseases. This review focuses on the mechanism of OS and a series of female reproductive processes, explaining the role of OS in female reproduction and female reproductive diseases caused by OS, including polycystic ovary syndrome (PCOS), endometriosis, preeclampsia and so on. Many signaling pathways involved in female reproduction, including the Keap1-Nrf2, NF-κB, FOXO and MAPK pathways, which are affected by OS, are described, providing new ideas for the mechanism of reproductive diseases.
Keywords: Antioxidants; Female fertility; Imbalance; Oxidative stress; ROS; Reproductive diseases; Signaling pathways.
Conflict of interest statement
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures

References
-
- Attaran M, Pasqualotto E, Falcone T, Goldberg JM, Miller KF, Agarwal A, Sharma RK. The effect of follicular fluid reactive oxygen species on the outcome of in vitro fertilization. International Journal of Fertility and Womens Medicine. 2000;45:314–320. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

