Bioelectronic Approaches to Control Neuroimmune Interactions in Acute Kidney Injury
- PMID: 30126836
- PMCID: PMC6546041
- DOI: 10.1101/cshperspect.a034231
Bioelectronic Approaches to Control Neuroimmune Interactions in Acute Kidney Injury
Abstract
Recent studies have shown renal protective effects of bioelectric approaches, including ultrasound treatment, electrical vagus nerve stimulation, and optogenetic brainstem C1 neuron stimulation. The renal protection acquired by all three modalities was lost in splenectomized mice and/or α7 subunit of the nicotinic acetylcholine receptor-deficient mice. C1 neuron-mediated renal protection was blocked by β2-adrenergic receptor antagonist. These findings indicate that all three methods commonly, at least partially, activate the cholinergic anti-inflammatory pathway, a well-studied neuroimmune pathway. In this article, we summarize the current understanding of neuroimmune axis-mediated kidney protection in preclinical models of acute kidney injury by these three modalities. Examination of the differences among these three modalities might lead to a further elucidation of the neuroimmune axis involved in renal protection and is of interest for developing new therapeutic approaches.
Copyright © 2019 Cold Spring Harbor Laboratory Press; all rights reserved.
Figures

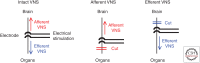



References
-
- Bellinger DL, Lorton D, Hamill RW, Felten SY, Felten DL. 1993. Acetylcholinesterase staining and choline acetyltransferase activity in the young adult rat spleen: Lack of evidence for cholinergic innervation. Brain Behav Immun 7: 191–204. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
