Histopathology of Bordetella pertussis in the Baboon Model
- PMID: 30126900
- PMCID: PMC6204705
- DOI: 10.1128/IAI.00511-18
Histopathology of Bordetella pertussis in the Baboon Model
Abstract
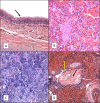
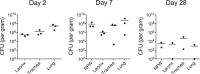
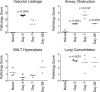
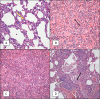
Pertussis is a severe respiratory disease caused by Bordetella pertussis The classic symptoms of pertussis include paroxysmal coughing with an inspiratory whoop, posttussive vomiting, cyanosis, and persistent coryzal symptoms. Infants under 2 months of age experience more severe disease, with most deaths occurring in this age group. Most of what is known about the pathology of pertussis in humans is from the evaluation of fatal human infant cases. The baboon model of pertussis provides the opportunity to evaluate the histopathology of severe but nonfatal pertussis. The baboon model recapitulates the characteristic clinical signs of pertussis observed in humans, including leukocytosis, paroxysmal coughing, mucus production, heavy colonization of the airway, and transmission of the bacteria between hosts. As in humans, baboons demonstrate age-related differences in clinical presentation, with younger animals experiencing more severe disease. We examined the histopathology of 5- to 6-week-old baboons, with the findings being similar to those reported for fatal human infant cases. In juvenile baboons, we found that the disease is highly inflammatory and concentrated to the lungs with signs of disease that would typically be diagnosed as acute respiratory distress syndrome (ARDS) and bronchopneumonia. In contrast, no significant pathology was observed in the trachea. Histopathological changes in the trachea were limited to cellular infiltrates and mucus production. Immunohistostaining revealed that the bacteria were localized to the surface of the ciliated epithelium in the conducting airways. Our observations provide important insights into the pathology of pertussis in typical, severe but nonfatal pertussis cases in a very relevant animal model.
Keywords: Bordetella pertussis; Papio; baboon; bronchiolitis; histopathology; infant; infectious disease; juvenile; nonhuman primates; pneumonia.
Copyright © 2018 American Society for Microbiology.
Figures




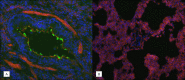
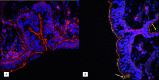
Similar articles
-
Pertussis disease and transmission and host responses: insights from the baboon model of pertussis.J Infect. 2017 Jun;74 Suppl 1:S114-S119. doi: 10.1016/S0163-4453(17)30201-3. J Infect. 2017. PMID: 28646950 Review.
-
In vivo imaging of bacterial colonization of the lower respiratory tract in a baboon model of Bordetella pertussis infection and transmission.Sci Rep. 2018 Aug 16;8(1):12297. doi: 10.1038/s41598-018-30896-7. Sci Rep. 2018. PMID: 30115990 Free PMC article.
-
Pathology and pathogenesis of fatal Bordetella pertussis infection in infants.Clin Infect Dis. 2008 Aug 1;47(3):328-38. doi: 10.1086/589753. Clin Infect Dis. 2008. PMID: 18558873
-
Fatal Pertussis in the Neonatal Mouse Model Is Associated with Pertussis Toxin-Mediated Pathology beyond the Airways.Infect Immun. 2017 Oct 18;85(11):e00355-17. doi: 10.1128/IAI.00355-17. Print 2017 Nov. Infect Immun. 2017. PMID: 28784932 Free PMC article.
-
Bordetella pertussis transmission.Pathog Dis. 2015 Nov;73(8):ftv068. doi: 10.1093/femspd/ftv068. Epub 2015 Sep 14. Pathog Dis. 2015. PMID: 26374235 Free PMC article. Review.
Cited by
-
Etiological distribution of pertussis-like syndrome in 756 children in Chengdu.Transl Pediatr. 2021 Apr;10(4):984-989. doi: 10.21037/tp-21-140. Transl Pediatr. 2021. PMID: 34012846 Free PMC article.
-
DNA-Dependent Interferon Induction and Lung Inflammation in Bordetella pertussis Infection.J Interferon Cytokine Res. 2023 Oct;43(10):478-486. doi: 10.1089/jir.2023.0066. Epub 2023 Aug 31. J Interferon Cytokine Res. 2023. PMID: 37651198 Free PMC article.
-
cAMP signaling of Bordetella adenylate cyclase toxin blocks M-CSF triggered upregulation of iron acquisition receptors on differentiating CD14+ monocytes.mSphere. 2024 Aug 28;9(8):e0040724. doi: 10.1128/msphere.00407-24. Epub 2024 Jul 30. mSphere. 2024. PMID: 39078132 Free PMC article.
-
Nonhuman Primates in Translational Research.Annu Rev Anim Biosci. 2022 Feb 15;10:441-468. doi: 10.1146/annurev-animal-021419-083813. Annu Rev Anim Biosci. 2022. PMID: 35167321 Free PMC article. Review.
-
Multivalent mRNA-DTP vaccines are immunogenic and provide protection from Bordetella pertussis challenge in mice.NPJ Vaccines. 2024 Jun 10;9(1):103. doi: 10.1038/s41541-024-00890-4. NPJ Vaccines. 2024. PMID: 38858423 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

