Testosterone Decreases House Dust Mite-Induced Type 2 and IL-17A-Mediated Airway Inflammation
- PMID: 30127088
- PMCID: PMC6143420
- DOI: 10.4049/jimmunol.1800293
Testosterone Decreases House Dust Mite-Induced Type 2 and IL-17A-Mediated Airway Inflammation
Abstract
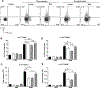
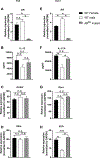
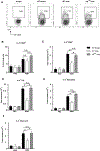
As adults, women are twice as likely as men to have asthma; however, the mechanisms explaining this sexual dimorphism remain unclear. Increased type 2 cytokines and/or IL-17A, leading to increased airway eosinophils and neutrophils, respectively, are associated with asthma. Previous studies showed that testosterone, signaling through the androgen receptor (AR), decreased Th2-mediated allergic inflammation and type 2 innate immune responses during allergic inflammation. Therefore, we hypothesized that testosterone and AR signaling attenuate type 2 and IL-17A-mediated airway inflammation. To test our hypothesis, sham-operated and gonadectomized female and male mice were intranasally challenged with house dust mite (HDM) or vehicle (PBS) for 3 wk. Testosterone decreased and ovarian hormones increased HDM-induced eosinophilic and neutrophilic inflammation, IgE production, and airway hyperresponsiveness, as well as decreased the numbers of IL-13+ CD4 Th2 cells and IL-17A+ CD4 Th17 cells in the lung. Next, using wild-type male and female mice and ARtfm male mice that are unable to signal through the AR, we determined AR signaling intrinsically attenuated IL-17A+ Th17 cells but indirectly decreased IL-13+ CD4 Th2 cells in the lung by suppressing HDM-induced IL-4 production. In vitro Th2 and Th17 differentiation experiments showed AR signaling had no direct effect on Th2 cell differentiation but decreased IL-17A protein expression and IL-23R mRNA relative expression from Th17 cells. Combined, these findings show AR signaling attenuated type 2 and IL-17A inflammation through different mechanisms and provide a potential explanation for the increased prevalence of asthma in women compared with men.
Copyright © 2018 by The American Association of Immunologists, Inc.
Figures

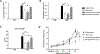



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

