TGF-β Signaling in Lung Health and Disease
- PMID: 30127261
- PMCID: PMC6121238
- DOI: 10.3390/ijms19082460
TGF-β Signaling in Lung Health and Disease
Abstract
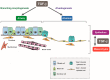
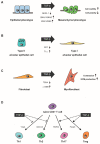
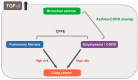
Transforming growth factor (TGF)-β is an evolutionarily conserved pleiotropic factor that regulates a myriad of biological processes including development, tissue regeneration, immune responses, and tumorigenesis. TGF-β is necessary for lung organogenesis and homeostasis as evidenced by genetically engineered mouse models. TGF-β is crucial for epithelial-mesenchymal interactions during lung branching morphogenesis and alveolarization. Expression and activation of the three TGF-β ligand isoforms in the lungs are temporally and spatially regulated by multiple mechanisms. The lungs are structurally exposed to extrinsic stimuli and pathogens, and are susceptible to inflammation, allergic reactions, and carcinogenesis. Upregulation of TGF-β ligands is observed in major pulmonary diseases, including pulmonary fibrosis, emphysema, bronchial asthma, and lung cancer. TGF-β regulates multiple cellular processes such as growth suppression of epithelial cells, alveolar epithelial cell differentiation, fibroblast activation, and extracellular matrix organization. These effects are closely associated with tissue remodeling in pulmonary fibrosis and emphysema. TGF-β is also central to T cell homeostasis and is deeply involved in asthmatic airway inflammation. TGF-β is the most potent inducer of epithelial-mesenchymal transition in non-small cell lung cancer cells and is pivotal to the development of tumor-promoting microenvironment in the lung cancer tissue. This review summarizes and integrates the current knowledge of TGF-β signaling relevant to lung health and disease.
Keywords: TGF-β; bronchial asthma; emphysema; lung cancer; pulmonary fibrosis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

