The Morphology and Assembly of Respiratory Syncytial Virus Revealed by Cryo-Electron Tomography
- PMID: 30127286
- PMCID: PMC6116276
- DOI: 10.3390/v10080446
The Morphology and Assembly of Respiratory Syncytial Virus Revealed by Cryo-Electron Tomography
Abstract
Human respiratory syncytial virus (RSV) is the leading cause of lower respiratory tract disease in young children. With repeat infections throughout life, it can also cause substantial disease in the elderly and in adults with compromised cardiac, pulmonary and immune systems. RSV is a pleomorphic enveloped RNA virus in the Pneumoviridae family. Recently, the three-dimensional (3D) structure of purified RSV particles has been elucidated, revealing three distinct morphological categories: spherical, asymmetric, and filamentous. However, the native 3D structure of RSV particles associated with or released from infected cells has yet to be investigated. In this study, we have established an optimized system for studying RSV structure by imaging RSV-infected cells on transmission electron microscopy (TEM) grids by cryo-electron tomography (cryo-ET). Our results demonstrate that RSV is filamentous across several virus strains and cell lines by cryo-ET, cryo-immuno EM, and thin section TEM techniques. The viral filament length varies from 0.5 to 12 μm and the average filament diameter is approximately 130 nm. Taking advantage of the whole cell tomography technique, we have resolved various stages of RSV assembly. Collectively, our results can facilitate the understanding of viral morphogenesis in RSV and other pleomorphic enveloped viruses.
Keywords: cryo-electron microscopy (cryo-EM); cryo-electron tomography (cryo-ET); enveloped virus; human respiratory syncytial virus (RSV); viral morphogenesis; virus assembly.
Conflict of interest statement
Martin L. Moore is an employee of Meissa Vaccines, Inc. Other authors declare no competing financial interests.
Figures
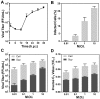



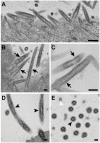

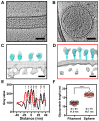
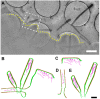

References
-
- Lamb R.A., Parks G.D. Paramyxoviridae: The viruses and their replication. In: Knipe D.M., Howley P.M., editors. Fields Virology. 5th ed. Volume 1. Wolters Kluwer/Lippincott Williams & Wilkins; Philadelphia, PA, USA: 2007. pp. 1449–1496.
-
- Nair H., Nokes D.J., Gessner B.D., Dherani M., Madhi S.A., Singleton R.J., O’Brien K.L., Roca A., Wright P.F., Bruce N., et al. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: A systematic review and meta-analysis. Lancet. 2010;375:1545–1555. doi: 10.1016/S0140-6736(10)60206-1. - DOI - PMC - PubMed
-
- Shi T., McAllister D.A., O’Brien K.L., Simoes E.A.F., Madhi S.A., Gessner B.D., Polack F.P., Balsells E., Acacio S., Aguayo C., et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in young children in 2015: A systematic review and modelling study. Lancet. 2017;390:946–958. doi: 10.1016/S0140-6736(17)30938-8. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

