The Emerging Role of Pathogenesis of IgA Nephropathy
- PMID: 30127305
- PMCID: PMC6112037
- DOI: 10.3390/jcm7080225
The Emerging Role of Pathogenesis of IgA Nephropathy
Abstract
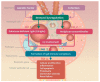
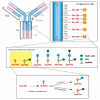
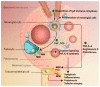
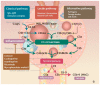
IgA nephropathy is an autoimmune disease induced by fthe ormation of galactose-deficient IgA1 and anti-glycans autoantibody. A multi-hit hypothesis was promoted to explain full expression of IgA nephropathy. The deposition of immune complex resulted in activation of the complement, increasing oxidative stress, promoting inflammatory cascade, and inducing cell apoptosis via mesangio-podocytic-tubular crosstalk. The interlinked signaling pathways of immune-complex-mediated inflammation can offer a novel target for therapeutic approaches. Treatments of IgA nephropathy are also summarized in our review article. In this article, we provide an overview of the recent basic and clinical studies in cell molecular regulation of IgAN for further treatment interventions.
Keywords: IgA nephropathy; anti-glycans autoantibody; galactose-deficient IgA1; inflammation; mesangio-podocytic-tubular crosstalk.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Silva F.G. Disappearance of glomerular mesangial IgA deposits after renal allograft transplantation. Transplantation. 1982;33:241–246. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

