JHU-083 selectively blocks glutaminase activity in brain CD11b+ cells and prevents depression-associated behaviors induced by chronic social defeat stress
- PMID: 30127344
- PMCID: PMC6372721
- DOI: 10.1038/s41386-018-0177-7
JHU-083 selectively blocks glutaminase activity in brain CD11b+ cells and prevents depression-associated behaviors induced by chronic social defeat stress
Erratum in
-
Correction: JHU-083 selectively blocks glutaminase activity in brain CD11b+ cells and prevents depression-associated behaviors induced by chronic social defeat stress.Neuropsychopharmacology. 2019 May;44(6):1178. doi: 10.1038/s41386-019-0354-3. Epub 2019 Mar 13. Neuropsychopharmacology. 2019. PMID: 30862949 Free PMC article.
Abstract
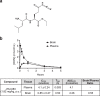
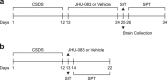
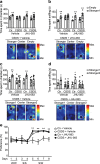
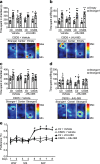
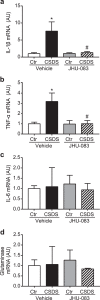
There are a number of clinically effective treatments for stress-associated psychiatric diseases, including major depressive disorder (MDD). Nonetheless, many patients exhibit resistance to first-line interventions calling for novel interventions based on pathological mechanisms. Accumulating evidence implicates altered glutamate signaling in MDD pathophysiology, suggesting that modulation of glutamate signaling cascades may offer novel therapeutic potential. Here we report that JHU-083, our recently developed prodrug of the glutaminase inhibitor 6-diazo-5-oxo-L-norleucine (DON) ameliorates social avoidance and anhedonia-like behaviors in mice subjected to chronic social defeat stress (CSDS). JHU-083 normalized CSDS-induced increases in glutaminase activity specifically in microglia-enriched CD11b+ cells isolated from the prefrontal cortex and hippocampus. JHU-083 treatment also reverses the CSDS-induced inflammatory activation of CD11b+ cells. These results support the importance of altered glutamate signaling in the behavioral abnormalities observed in the CSDS model, and identify glutaminase in microglia-enriched CD11b+ cells as a pharmacotherapeutic target implicated in the pathophysiology of stress-associated psychiatric conditions such as MDD.
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Glutamine Antagonist JHU-083 Normalizes Aberrant Hippocampal Glutaminase Activity and Improves Cognition in APOE4 Mice.J Alzheimers Dis. 2020;77(1):437-447. doi: 10.3233/JAD-190588. J Alzheimers Dis. 2020. PMID: 32675407 Free PMC article.
-
Effect of Toll-like receptor 4 on depressive-like behaviors induced by chronic social defeat stress.Brain Behav. 2020 Mar;10(3):e01525. doi: 10.1002/brb3.1525. Epub 2020 Jan 16. Brain Behav. 2020. PMID: 31945269 Free PMC article.
-
Ginsenoside Rg1 ameliorates chronic social defeat stress-induced depressive-like behaviors and hippocampal neuroinflammation.Life Sci. 2020 Jul 1;252:117669. doi: 10.1016/j.lfs.2020.117669. Epub 2020 Apr 13. Life Sci. 2020. PMID: 32298740
-
Phytoestrogen genistein modulates neuron-microglia signaling in a mouse model of chronic social defeat stress.Neuropharmacology. 2022 Mar 15;206:108941. doi: 10.1016/j.neuropharm.2021.108941. Epub 2022 Jan 3. Neuropharmacology. 2022. PMID: 34990615
-
Inflamed brain: Targeting immune changes and inflammation for treatment of depression.Psychiatry Clin Neurosci. 2021 Oct;75(10):304-311. doi: 10.1111/pcn.13286. Epub 2021 Jul 28. Psychiatry Clin Neurosci. 2021. PMID: 34227186 Free PMC article. Review.
Cited by
-
The glutamine antagonist prodrug JHU-083 slows malignant glioma growth and disrupts mTOR signaling.Neurooncol Adv. 2020 Oct 29;3(1):vdaa149. doi: 10.1093/noajnl/vdaa149. eCollection 2021 Jan-Dec. Neurooncol Adv. 2020. PMID: 33681764 Free PMC article.
-
MRI demonstrates glutamine antagonist-mediated reversal of cerebral malaria pathology in mice.Proc Natl Acad Sci U S A. 2018 Dec 18;115(51):E12024-E12033. doi: 10.1073/pnas.1812909115. Epub 2018 Dec 4. Proc Natl Acad Sci U S A. 2018. PMID: 30514812 Free PMC article.
-
Microglial phagocytosis and regulatory mechanisms after stroke.J Cereb Blood Flow Metab. 2022 Sep;42(9):1579-1596. doi: 10.1177/0271678X221098841. Epub 2022 May 1. J Cereb Blood Flow Metab. 2022. PMID: 35491825 Free PMC article.
-
Causal impact of local inflammation in the nasal cavity on higher brain function and cognition.Neurosci Res. 2021 Nov;172:110-115. doi: 10.1016/j.neures.2021.04.009. Epub 2021 Apr 28. Neurosci Res. 2021. PMID: 33932551 Free PMC article.
-
Glutamine antagonism attenuates physical and cognitive deficits in a model of MS.Neurol Neuroimmunol Neuroinflamm. 2019 Aug 29;6(6):e609. doi: 10.1212/NXI.0000000000000609. Print 2019 Nov. Neurol Neuroimmunol Neuroinflamm. 2019. PMID: 31467038 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

