Iron restriction inside macrophages regulates pulmonary host defense against Rhizopus species
- PMID: 30127354
- PMCID: PMC6102248
- DOI: 10.1038/s41467-018-05820-2
Iron restriction inside macrophages regulates pulmonary host defense against Rhizopus species
Erratum in
-
Author Correction: Iron restriction inside macrophages regulates pulmonary host defense against Rhizopus species.Nat Commun. 2018 Nov 22;9(1):5015. doi: 10.1038/s41467-018-07301-y. Nat Commun. 2018. PMID: 30467313 Free PMC article.
Abstract
Mucormycosis is a life-threatening respiratory fungal infection predominantly caused by Rhizopus species. Mucormycosis has incompletely understood pathogenesis, particularly how abnormalities in iron metabolism compromise immune responses. Here we show how, as opposed to other filamentous fungi, Rhizopus spp. establish intracellular persistence inside alveolar macrophages (AMs). Mechanistically, lack of intracellular swelling of Rhizopus conidia results in surface retention of melanin, which induces phagosome maturation arrest through inhibition of LC3-associated phagocytosis. Intracellular inhibition of Rhizopus is an important effector mechanism, as infection of immunocompetent mice with swollen conidia, which evade phagocytosis, results in acute lethality. Concordantly, AM depletion markedly increases susceptibility to mucormycosis. Host and pathogen transcriptomics, iron supplementation studies, and genetic manipulation of iron assimilation of fungal pathways demonstrate that iron restriction inside macrophages regulates immunity against Rhizopus. Our findings shed light on the pathogenetic mechanisms of mucormycosis and reveal the role of macrophage-mediated nutritional immunity against filamentous fungi.
Conflict of interest statement
The authors declare no competing interests.
Figures

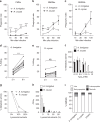

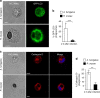

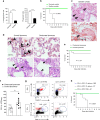
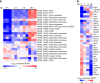

References
-
- Kontoyiannis DP, et al. Prospective surveillance for invasive fungal infections in hematopoietic stem cell transplant recipients, 2001–2006: overview of the Transplant-Associated Infection Surveillance Network (TRANSNET) Database. Clin. Infect. Dis. 2010;50:1091–1100. doi: 10.1086/651263. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

