Endogenous glucocorticoids control host resistance to viral infection through the tissue-specific regulation of PD-1 expression on NK cells
- PMID: 30127438
- PMCID: PMC6138242
- DOI: 10.1038/s41590-018-0185-0
Endogenous glucocorticoids control host resistance to viral infection through the tissue-specific regulation of PD-1 expression on NK cells
Abstract
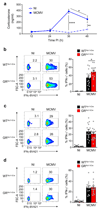
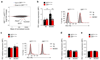
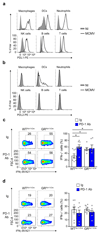
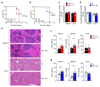
Controlling the balance between immunity and immunopathology is crucial for host resistance to pathogens. After infection, activation of the hypothalamic-pituitary-adrenal (HPA) axis leads to the production of glucocorticoids. However, the pleiotropic effects of these steroid hormones make it difficult to delineate their precise role(s) in vivo. Here we found that the regulation of natural killer (NK) cell function by the glucocorticoid receptor (GR) was required for host survival after infection with mouse cytomegalovirus (MCMV). Mechanistically, endogenous glucocorticoids produced shortly after infection induced selective and tissue-specific expression of the checkpoint receptor PD-1 on NK cells. This glucocorticoid-PD-1 pathway limited production of the cytokine IFN-γ by spleen NK cells, which prevented immunopathology. Notably, this regulation did not compromise viral clearance. Thus, the fine tuning of NK cell functions by the HPA axis preserved tissue integrity without impairing pathogen elimination, which reveals a novel aspect of neuroimmune regulation.
Conflict of interest statement
EV is a cofounder and employee of Innate Pharma. The other authors declare no competing financial interests.
Figures


Comment in
-
Glucocorticoids and NK cell PD-1.Nat Immunol. 2018 Sep;19(9):908-910. doi: 10.1038/s41590-018-0190-3. Nat Immunol. 2018. PMID: 30127435 No abstract available.
-
The brain-immune cells axis controls tissue specific immunopathology.Cell Mol Immunol. 2019 Feb;16(2):101-103. doi: 10.1038/s41423-018-0176-y. Epub 2018 Nov 7. Cell Mol Immunol. 2019. PMID: 30405148 Free PMC article.
-
Neuroendocrine control of natural killer cell function through the checkpoint molecule PD-1.Cell Mol Immunol. 2019 Feb;16(2):109-111. doi: 10.1038/s41423-018-0180-2. Epub 2018 Nov 22. Cell Mol Immunol. 2019. PMID: 30467417 Free PMC article. No abstract available.
-
Commentary: Endogenous glucocorticoids control host resistance to viral infection through tissue-specific regulation of PD-1 expression on NK cells.Cell Mol Immunol. 2019 Mar;16(3):203-204. doi: 10.1038/s41423-018-0190-0. Epub 2018 Dec 12. Cell Mol Immunol. 2019. PMID: 30542143 Free PMC article. No abstract available.
References
-
- Webster JI, Tonelli L, Sternberg EM. Neuroendocrine regulation of immunity. Annu Rev Immunol. 2002;20:125–163. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

