Histone Deacetylase 7-Derived Peptides Play a Vital Role in Vascular Repair and Regeneration
- PMID: 30128229
- PMCID: PMC6097091
- DOI: 10.1002/advs.201800006
Histone Deacetylase 7-Derived Peptides Play a Vital Role in Vascular Repair and Regeneration
Abstract
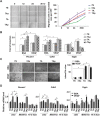
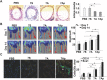
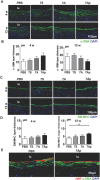
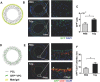
Cardiovascular disease is a leading cause of morbidity and mortality globally. Accumulating evidence indicates that local resident stem/progenitor cells play an important role in vascular regeneration. Recently, it is demonstrated that a histone deacetylase 7-derived 7-amino acid peptide (7A, MHSPGAD) is critical in modulating the mobilization and orientated differentiation of these stem/progenitor cells. Here, its therapeutic efficacy in vascular repair and regeneration is evaluated. In vitro functional analyses reveal that the 7A peptide, in particular phosphorylated 7A (7Ap, MH[pSer]PGAD), could increase stem cell antigen-1 positive (Sca1+) vascular progenitor cell (VPC) migration and differentiation toward an endothelial cell lineage. Furthermore, local delivery of 7A as well as 7Ap could enhance angiogenesis and ameliorate vascular injury in ischaemic tissues; these findings are confirmed in a femoral artery injury model and a hindlimb ischaemia model, respectively. Importantly, sustained delivery of 7A, especially 7Ap, from tissue-engineered vascular grafts could attract Sca1+-VPC cells into the grafts, contributing to endothelialization and intima/media formation in the vascular graft. These results suggest that this novel type of peptides has great translational potential in vascular regenerative medicine.
Keywords: HDAC7‐derived peptide; ischaemia disease; tissue‐engineered vascular grafts (TEVGs); vascular progenitor cells (VPCs).
Figures




Similar articles
-
A histone deacetylase 7-derived peptide promotes vascular regeneration via facilitating 14-3-3γ phosphorylation.Stem Cells. 2020 Apr;38(4):556-573. doi: 10.1002/stem.3122. Epub 2020 Jan 29. Stem Cells. 2020. PMID: 31721359 Free PMC article.
-
A collagen hydrogel loaded with HDAC7-derived peptide promotes the regeneration of infarcted myocardium with functional improvement in a rodent model.Acta Biomater. 2019 Mar 1;86:223-234. doi: 10.1016/j.actbio.2019.01.022. Epub 2019 Jan 16. Acta Biomater. 2019. PMID: 30660010
-
Histone Deacetylase 7-Derived 7-Amino Acid Peptide Increases Skin Wound Healing via Regulating Epidermal Fibroblast Proliferation and Migration.J Cell Mol Med. 2024 Nov;28(22):e70209. doi: 10.1111/jcmm.70209. J Cell Mol Med. 2024. PMID: 39601342 Free PMC article.
-
Vascular Stem/Progenitor Cell Migration and Differentiation in Atherosclerosis.Antioxid Redox Signal. 2018 Jul 10;29(2):219-235. doi: 10.1089/ars.2017.7171. Epub 2017 Jul 5. Antioxid Redox Signal. 2018. PMID: 28537424 Review.
-
Vascular repair by endothelial progenitor cells.Cardiovasc Res. 2008 Jun 1;78(3):413-21. doi: 10.1093/cvr/cvn081. Epub 2008 Mar 18. Cardiovasc Res. 2008. PMID: 18349136 Review.
Cited by
-
Pharmacological manipulation of macrophage autophagy effectively rejuvenates the regenerative potential of biodegrading vascular graft in aging body.Bioact Mater. 2021 Oct 20;11:283-299. doi: 10.1016/j.bioactmat.2021.09.027. eCollection 2022 May. Bioact Mater. 2021. PMID: 34977432 Free PMC article.
-
A biomimetic orthogonal-bilayer tubular scaffold for the co-culture of endothelial cells and smooth muscle cells.RSC Adv. 2021 Sep 27;11(50):31783-31790. doi: 10.1039/d1ra04472a. eCollection 2021 Sep 21. RSC Adv. 2021. PMID: 35496878 Free PMC article.
-
Biomaterial-based vascularization strategies for enhanced treatment of peripheral arterial disease.J Nanobiotechnology. 2025 Feb 12;23(1):103. doi: 10.1186/s12951-025-03140-4. J Nanobiotechnology. 2025. PMID: 39940018 Free PMC article. Review.
-
Nitrate-Functionalized poly(ε-Caprolactone) Small-Diameter Vascular Grafts Enhance Vascular Regeneration via Sustained Release of Nitric Oxide.Front Bioeng Biotechnol. 2021 Nov 30;9:770121. doi: 10.3389/fbioe.2021.770121. eCollection 2021. Front Bioeng Biotechnol. 2021. PMID: 34917597 Free PMC article.
-
Mechanically reinforced biotubes for arterial replacement and arteriovenous grafting inspired by architectural engineering.Sci Adv. 2022 Mar 18;8(11):eabl3888. doi: 10.1126/sciadv.abl3888. Epub 2022 Mar 16. Sci Adv. 2022. PMID: 35294246 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
