Monoclonal antibodies from a patient with anti-NMDA receptor encephalitis
- PMID: 30128318
- PMCID: PMC6093837
- DOI: 10.1002/acn3.592
Monoclonal antibodies from a patient with anti-NMDA receptor encephalitis
Abstract
Objective: Anti-NMDA receptor encephalitis (ANRE) is a potentially lethal encephalitis attributed to autoantibodies against the N-methyl-D-aspartate receptor (NMDAR). We sought to clone and characterize monoclonal antibodies (mAbs) from an ANRE patient.
Methods: We used a hybridoma method to clone two IgG mAbs from a female patient with ANRE without teratoma, and characterized their binding activities on NMDAR-transfected cell lines, cultured primary rat neurons, and mouse hippocampus. We also assessed their effects on voluntary locomotor activity in mice and binding to NMDAR in vivo.
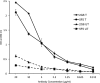
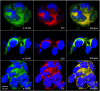
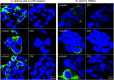
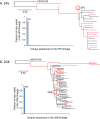
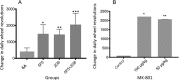
Results: The mAbs are structurally distinct and arose from distinct B-cell lineages. They recognize different epitopes on the GluN1 amino terminal domain (ATD), yet both require amino acids important for post-translational modification. Both mAbs bind subsets of GluN1 on cultured rat hippocampal neurons. The 5F5 mAb binds mouse brain hippocampal tissues, and the GluN1 recognized on cultured rat neurons was substantially extra-synaptic. Antibody binding to primary hippocampal neurons induced receptor internalization. The NMDAR inhibitor MK-801 inhibited internalization without preventing mAb binding; AP5 inhibited both mAb binding and internalization. Exposure of mice to the mAbs following permeabilization of the blood brain barrier increased voluntary wheel running activity, similar to low doses of the NMDAR inhibitor, MK-801.
Interpretation: These mAbs recapitulate features demonstrated in previous studies of ANRE patient CSF, and exert effects on NMDAR in vitro and in vivo consistent with modulation of NMDAR activity.
Figures








References
-
- Zhang Q, Tanaka K, Sun P, et al. Suppression of synaptic plasticity by cerebrospinal fluid from anti‐NMDA receptor encephalitis patients. Neurobiol Dis 2012;45:610–615. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

