Knockdown of Mns1 Increases Susceptibility to Craniofacial Defects Following Gastrulation-Stage Alcohol Exposure in Mice
- PMID: 30129265
- PMCID: PMC6214710
- DOI: 10.1111/acer.13876
Knockdown of Mns1 Increases Susceptibility to Craniofacial Defects Following Gastrulation-Stage Alcohol Exposure in Mice
Abstract
Background: MNS1 (meiosis-specific nuclear structural protein 1) is necessary for motile cilia function, such as sperm flagella or those found in the embryonic primitive node. While little is known regarding the function or expression pattern of MNS1 in the embryo, co-immunoprecipitation experiments in sperm have determined that MNS1 interacts with ciliary proteins, which are also important during development. Establishment of morphogenic gradients is dependent on normal ciliary motion in the primitive node beginning during gastrulation (gestational day [GD] 7 in the mouse, second-third week of pregnancy in humans), a critical window for face, eye, and brain development and particularly susceptible to perturbations of developmental signals. The current study investigates the role of Mns1 in craniofacial defects associated with gastrulation-stage alcohol exposure.
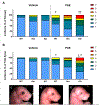
Methods: On GD7, pregnant Mns1+/- dams were administered 2 doses of ethanol (5.8 g/kg total) or vehicle 4 hours apart to target gastrulation. On GD17, fetuses were examined for ocular defects by scoring each eye on a scale from 1 to 7 (1 = normal, 2 to 7 = defects escalating in severity). Craniofacial and brain abnormalities were also assessed.
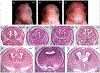
Results: Prenatal alcohol exposure (PAE) significantly increased the rate of defects in wild-type fetuses, as PAE fetuses had an incidence rate of 41.18% compared to a 10% incidence rate in controls. Furthermore, PAE interacted with genotype to significantly increase the defect rate and severity in Mns1+/- (64.29%) and Mns1-/- mice (92.31%). PAE Mns1-/- fetuses with severe eye defects also presented with craniofacial dysmorphologies characteristic of fetal alcohol syndrome and midline tissue loss in the brain, palate, and nasal septum.
Conclusions: These data demonstrate that a partial or complete knockdown of Mns1 interacts with PAE to increase the susceptibility to ocular defects and correlating craniofacial and brain anomalies, likely though interaction of alcohol with motile cilia function. These results further our understanding of genetic risk factors that may underlie susceptibility to teratogenic exposures.
Keywords: Birth Defects; Development; Ethanol; Fetal Alcohol Syndrome; Transgenic Mice.
© 2018 by the Research Society on Alcoholism.
Figures


Similar articles
-
Efcab7 deletion sensitizes mice to the teratogenic effects of gastrulation-stage alcohol exposure.Reprod Toxicol. 2024 Dec;130:108729. doi: 10.1016/j.reprotox.2024.108729. Epub 2024 Oct 2. Reprod Toxicol. 2024. PMID: 39366525
-
Gastrulation-stage alcohol exposure induces similar rates of craniofacial malformations in male and female C57BL/6J mice.Birth Defects Res. 2024 Jan;116(1):e2292. doi: 10.1002/bdr2.2292. Epub 2023 Dec 20. Birth Defects Res. 2024. PMID: 38116840 Free PMC article.
-
Maternal oral intake mouse model for fetal alcohol spectrum disorders: ocular defects as a measure of effect.Alcohol Clin Exp Res. 2006 Oct;30(10):1791-8. doi: 10.1111/j.1530-0277.2006.00212.x. Alcohol Clin Exp Res. 2006. PMID: 17010146
-
The chick embryo as a model for the effects of prenatal exposure to alcohol on craniofacial development.Dev Biol. 2016 Jul 15;415(2):314-325. doi: 10.1016/j.ydbio.2016.01.007. Epub 2016 Jan 14. Dev Biol. 2016. PMID: 26777098 Review.
-
Overview of the Genetic Basis and Epigenetic Mechanisms that Contribute to FASD Pathobiology.Curr Top Med Chem. 2017;17(7):808-828. doi: 10.2174/1568026616666160414124816. Curr Top Med Chem. 2017. PMID: 27086780 Review.
Cited by
-
The role of HnrnpF/H as a driver of oligoteratozoospermia.iScience. 2024 Jun 6;27(7):110198. doi: 10.1016/j.isci.2024.110198. eCollection 2024 Jul 19. iScience. 2024. PMID: 39092172 Free PMC article.
-
N-Acetylcysteine prevents the decreases in cardiac collagen I/III ratio and systolic function in neonatal mice with prenatal alcohol exposure.Toxicol Lett. 2019 Oct 15;315:87-95. doi: 10.1016/j.toxlet.2019.08.010. Epub 2019 Aug 16. Toxicol Lett. 2019. PMID: 31425726 Free PMC article.
-
Effect of Embryonic Alcohol Exposure on Craniofacial and Skin Melanocyte Development: Insights from Zebrafish (Danio rerio).Toxics. 2022 Sep 18;10(9):544. doi: 10.3390/toxics10090544. Toxics. 2022. PMID: 36136509 Free PMC article.
-
Gene-alcohol interactions in birth defects.Curr Top Dev Biol. 2023;152:77-113. doi: 10.1016/bs.ctdb.2022.10.003. Epub 2022 Nov 14. Curr Top Dev Biol. 2023. PMID: 36707215 Free PMC article.
-
Neuroimmune Interactions in Fetal Alcohol Spectrum Disorders: Potential Therapeutic Targets and Intervention Strategies.Cells. 2023 Sep 21;12(18):2323. doi: 10.3390/cells12182323. Cells. 2023. PMID: 37759545 Free PMC article. Review.
References
-
- Brocardo PS, Gil-Mohapel J, Christie BR (2011) The role of oxidative stress in fetal alcohol spectrum disorders. Brain research reviews 67:209–225. - PubMed
-
- Cook CS, Nowotny AZ, Sulik KK (1987) Fetal alcohol syndrome: eye malformations in a mouse model. Archives of Ophthalmology 105:1576–1581. - PubMed
-
- Cortés CR, Metzis V, Wicking C (2015) Unmasking the ciliopathies: craniofacial defects and the primary cilium. Wiley Interdisciplinary Reviews: Developmental Biology 4:637–653. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

