Generation and characterization of a high affinity anti-human FcRn antibody, rozanolixizumab, and the effects of different molecular formats on the reduction of plasma IgG concentration
- PMID: 30130439
- PMCID: PMC6291300
- DOI: 10.1080/19420862.2018.1505464
Generation and characterization of a high affinity anti-human FcRn antibody, rozanolixizumab, and the effects of different molecular formats on the reduction of plasma IgG concentration
Abstract
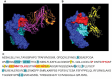
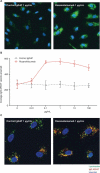
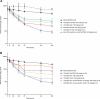
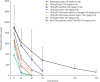
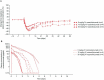
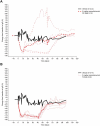
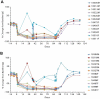
Rozanolixizumab (UCB7665), a humanized high-affinity anti-human neonatal Fc receptor (FcRn) monoclonal antibody (IgG4P), has been developed to reduce pathogenic IgG in autoimmune and alloimmune diseases. We document the antibody isolation and compare rozanolixizumab with the same variable region expressed in various mono-, bi- and trivalent formats. We report activity data for rozanolixizumab and the different molecular formats in human cells, FcRn-transgenic mice, and cynomolgus monkeys. Rozanolixizumab, considered the most effective molecular format, dose-dependently and selectively reduced plasma IgG concentrations in an FcRn-transgenic mouse model (no effect on albumin). Intravenous (IV) rozanolixizumab dosing in cynomolgus monkeys demonstrated non-linear pharmacokinetics indicative of target-mediated drug disposition; single IV rozanolixizumab doses (30 mg/kg) in cynomolgus monkeys reduced plasma IgG concentration by 69% by Day 7 post-administration. Daily IV administration of rozanolixizumab (initial 30 mg/kg loading dose; 5 mg/kg daily thereafter) reduced plasma IgG concentrations in all cynomolgus monkeys, with low concentrations maintained throughout the treatment period (42 days). In a 13-week toxicology study in cynomolgus monkeys, supra-pharmacological subcutaneous and IV doses of rozanolixizumab (≤ 150 mg/kg every 3 days) were well tolerated, inducing sustained (but reversible) reductions in IgG concentrations by up to 85%, with no adverse events observed. We have demonstrated accelerated natural catabolism of IgG through inhibition of IgG:FcRn interactions in mice and cynomolgus monkeys. Inhibition of FcRn with rozanolixizumab may provide a novel therapeutic approach to reduce pathogenic IgG in human autoimmune disease. Rozanolixizumab is being investigated in patients with immune thrombocytopenia (NCT02718716) and myasthenia gravis (NCT03052751).
Keywords: FcRn; FcRn blockade; IgG catabolism; UCB7665; autoantibody; autoimmunity; immune thrombocytopenia; myasthenia gravis; rozanolixizumab; target-mediated drug disposition.
Figures
References
-
- Schwartz J, Winters JL, Padmanabhan A, Balogun RA, Delaney M, Linenberger ML, Szczepiorkowski ZM, Williams ME, Wu Y, Shaz BH.. Guidelines on the use of therapeutic apheresis in clinical practice-evidence-based approach from the Writing Committee of the American Society for Apheresis: the sixth special issue. J Clin Apher. 2013;28:145–284. doi:10.1002/jca.21276. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
