Nuclear Folate Metabolism
- PMID: 30130467
- PMCID: PMC11788913
- DOI: 10.1146/annurev-nutr-071714-034441
Nuclear Folate Metabolism
Abstract
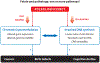
Despite unequivocal evidence that folate deficiency increases risk for human pathologies, and that folic acid intake among women of childbearing age markedly decreases risk for birth defects, definitive evidence for a causal biochemical pathway linking folate to disease and birth defect etiology remains elusive. The de novo and salvage pathways for thymidylate synthesis translocate to the nucleus of mammalian cells during S- and G2/M-phases of the cell cycle and associate with the DNA replication and repair machinery, which limits uracil misincorporation into DNA and genome instability. There is increasing evidence that impairments in nuclear de novo thymidylate synthesis occur in many pathologies resulting from impairments in one-carbon metabolism. Understanding the roles and regulation of nuclear de novo thymidylate synthesis and its relationship to genome stability will increase our understanding of the fundamental mechanisms underlying folate- and vitamin B12-associated pathologies.
Keywords: DNA synthesis; folate; neural tube defects; replitase; thymidylate.
Figures

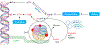

References
-
- Abali EE, Skacel NE, Celikkaya H, Hsieh YC. 2008. Regulation of human dihydrofolate reductase activity and expression. Vitam. Horm 79:267–92 - PubMed
-
- Ames BN. 2001. DNA damage from micronutrient deficiencies is likely to be a major cause of cancer. Mutat. Res 475:7–20 - PubMed
-
- An S, Kumar R, Sheets ED, Benkovic SJ. 2008. Reversible compartmentalization of de novo purine biosynthetic complexes in living cells. Science 320:103–6 - PubMed
-
- Andersen S, Heine T, Sneve R, Konig I, Krokan HE, et al. 2005. Incorporation of dUMP into DNA is a major source of spontaneous DNA damage, while excision of uracil is not required for cytotoxicity of fluoropyrimidines in mouse embryonic fibroblasts. Carcinogenesis 26:547–55 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

