Investigation of the effect of hepatic metabolism on off-target cardiotoxicity in a multi-organ human-on-a-chip system
- PMID: 30130706
- PMCID: PMC6126670
- DOI: 10.1016/j.biomaterials.2018.07.062
Investigation of the effect of hepatic metabolism on off-target cardiotoxicity in a multi-organ human-on-a-chip system
Abstract
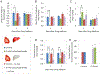
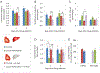
Regulation of cosmetic testing and poor predictivity of preclinical drug studies has spurred efforts to develop new methods for systemic toxicity. Current in vitro assays do not fully represent physiology, often lacking xenobiotic metabolism. Functional human multi-organ systems containing iPSC derived cardiomyocytes and primary hepatocytes were maintained under flow using a low-volume pumpless system in a serum-free medium. The functional readouts for contractile force and electrical conductivity enabled the non-invasive study of cardiac function. The presence of the hepatocytes in the system induced cardiotoxic effects from cyclophosphamide and reduced them for terfenadine due to drug metabolism, as expected from each compound's pharmacology. A computational fluid dynamics simulation enabled the prediction of terfenadine-fexofenadine pharmacokinetics, which was validated by HPLC-MS. This in vitro platform recapitulates primary aspects of the in vivo crosstalk between heart and liver and enables pharmacological studies, involving both organs in a single in vitro platform. The system enables non-invasive readouts of cardiotoxicity of drugs and their metabolites. Hepatotoxicity can also be evaluated by biomarker analysis and change in metabolic function. Integration of metabolic function in toxicology models can improve adverse effects prediction in preclinical studies and this system could also be used for chronic studies as well.
Keywords: Cardiotoxicity; Functional readouts; Human-on-a-chip; Metabolism; Non-invasive.
Copyright © 2018 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Disclosure of Potential Conflict of Interest
The authors confirm that competing financial interests exist but there has been no financial support for this research that could have influenced its outcome. However, JJH and MLS have a potential competing financial interest, in that a company has been formed to market services for types of cells like this in body-on-a-chip devices.
Figures







References
-
- Cook D, Brown D, Alexander R, March R, Morgan P, Satterthwaite G, Pangalos MN, Lessons learned from the fate of AstraZeneca’s drug pipeline: a five-dimensional framework, Nat Rev Drug Discov 13(6) (2014) 419–31. - PubMed
-
- Hutson MS, Alexander PG, Allwardt V, Aronoff DM, Bruner-Tran KL, Cliffel DE, Davidson JM, Gough A, Markov DA, McCawley LJ, McKenzie JR, McLean JA, Osteen KG, Pensabene V, Samson PC, Senutovitch NK, Sherrod SD, Shotwell MS, Taylor DL, Tetz LM, Tuan RS, Vernetti LA, Wikswo JP, Organs-on-Chips as Bridges for Predictive Toxicology, Applied In Vitro Toxicology 2(2) (2016) 97–102.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

