Reconstitution of a thermophilic Cu+ importer in vitro reveals intrinsic high-affinity slow transport driving accumulation of an essential metal ion
- PMID: 30131336
- PMCID: PMC6177576
- DOI: 10.1074/jbc.RA118.004802
Reconstitution of a thermophilic Cu+ importer in vitro reveals intrinsic high-affinity slow transport driving accumulation of an essential metal ion
Abstract
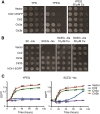
Acquisition of the trace element copper (Cu) is critical to drive essential eukaryotic processes such as oxidative phosphorylation, iron mobilization, peptide hormone biogenesis, and connective tissue maturation. The Ctr1/Ctr3 family of Cu importers, first discovered in fungi and conserved in mammals, are critical for Cu+ movement across the plasma membrane or mobilization from endosomal compartments. Whereas ablation of Ctr1 in mammals is embryonic lethal, and Ctr1 is critical for dietary Cu absorption, cardiac function, and systemic iron distribution, little is known about the intrinsic contribution of Ctr1 for Cu+ permeation through membranes or its mechanism of action. Here, we identify three members of a Cu+ importer family from the thermophilic fungus Chaetomium thermophilum: Ctr3a and Ctr3b, which function on the plasma membrane, and Ctr2, which likely functions in endosomal Cu mobilization. All three proteins drive Cu and isoelectronic silver (Ag) uptake in cells devoid of Cu+ importers. Transport activity depends on signature amino acid motifs that are conserved and essential for all Ctr1/3 transporters. Ctr3a is stable and amenable to purification and was incorporated into liposomes to reconstitute an in vitro Ag+ transport assay characterized by stopped-flow spectroscopy. Ctr3a has intrinsic high-affinity metal ion transport activity that closely reflects values determined in vivo, with slow turnover kinetics. Given structural models for mammalian Ctr1, Ctr3a likely functions as a low-efficiency Cu+ ion channel. The Ctr1/Ctr3 family may be tuned to import essential yet potentially toxic Cu+ ions at a slow rate to meet cellular needs, while minimizing labile intracellular Cu+ pools.
Keywords: Ctr1; copper; copper transport; liposome; metal homeostasis; thermophile.
© 2018 Logeman and Thiele.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures







References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

