Phosphoproteomic analysis of chimeric antigen receptor signaling reveals kinetic and quantitative differences that affect cell function
- PMID: 30131370
- PMCID: PMC6186424
- DOI: 10.1126/scisignal.aat6753
Phosphoproteomic analysis of chimeric antigen receptor signaling reveals kinetic and quantitative differences that affect cell function
Abstract
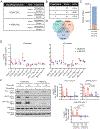
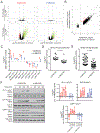
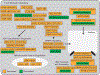
Chimeric antigen receptors (CARs) link an antigen recognition domain to intracellular signaling domains to redirect T cell specificity and function. T cells expressing CARs with CD28/CD3ζ or 4-1BB/CD3ζ signaling domains are effective at treating refractory B cell malignancies but exhibit differences in effector function, clinical efficacy, and toxicity that are assumed to result from the activation of divergent signaling cascades. We analyzed stimulation-induced phosphorylation events in primary human CD8+ CD28/CD3ζ and 4-1BB/CD3ζ CAR T cells by mass spectrometry and found that both CAR constructs activated similar signaling intermediates. Stimulation of CD28/CD3ζ CARs activated faster and larger-magnitude changes in protein phosphorylation, which correlated with an effector T cell-like phenotype and function. In contrast, 4-1BB/CD3ζ CAR T cells preferentially expressed T cell memory-associated genes and exhibited sustained antitumor activity against established tumors in vivo. Mutagenesis of the CAR CD28 signaling domain demonstrated that the increased CD28/CD3ζ CAR signal intensity was partly related to constitutive association of Lck with this domain in CAR complexes. Our data show that CAR signaling pathways cannot be predicted solely by the domains used to construct the receptor and that signal strength is a key determinant of T cell fate. Thus, tailoring CAR design based on signal strength may lead to improved clinical efficacy and reduced toxicity.
Copyright © 2018 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.
Conflict of interest statement
Competing Interests:
S.R.R. is a founder, shareholder, and scientific advisor of Juno Therapeutics. R.G. is a consultant for Juno Therapeutics. STII CAR technology used in this study is licensed to Juno Therapeutics. A.I.S. and S.R.R. have filed a provisional patent application number 62/635,450 covering applications of mutant CD28 CARs for cellular therapy. The other authors declare that they have no competing interests.
Figures




References
-
- Maude SL, Frey N, Shaw PA, Aplenc R, Barrett DM, Bunin NJ, Chew A, Gonzalez VE, Zheng Z, Lacey SF, Mahnke YD, Melenhorst JJ, Rheingold SR, Shen A, Teachey DT, Levine BL, June CH, Porter DL, Grupp SA, Chimeric antigen receptor T cells for sustained remissions in leukemia, N Engl J Med 371, 1507–1517 (2014). - PMC - PubMed
-
- Davila ML, Riviere I, Wang X, Bartido S, Park J, Curran K, Chung SS, Stefanski J, Borquez-Ojeda O, Olszewska M, Qu J, Wasielewska T, He Q, Fink M, Shinglot H, Youssif M, Satter M, Wang Y, Hosey J, Quintanilla H, Halton E, Bernal Y, Bouhassira DCG, Arcila ME, Gonen M, Roboz GJ, Maslak P, Douer D, Frattini MG, Giralt S, Sadelain M, Brentjens R, Efficacy and toxicity management of 19–28z CAR T cell therapy in B cell acute lymphoblastic leukemia, Sci Transl Med 6, 224–25 (2014). - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

