Tomato PEPR1 ORTHOLOG RECEPTOR-LIKE KINASE1 Regulates Responses to Systemin, Necrotrophic Fungi, and Insect Herbivory
- PMID: 30131419
- PMCID: PMC6181013
- DOI: 10.1105/tpc.17.00908
Tomato PEPR1 ORTHOLOG RECEPTOR-LIKE KINASE1 Regulates Responses to Systemin, Necrotrophic Fungi, and Insect Herbivory
Abstract
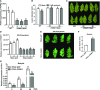
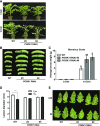
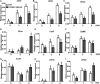
Endogenous peptides regulate plant immunity and growth. Systemin, a peptide specific to the Solanaceae, is known for its functions in plant responses to insect herbivory and pathogen infections. Here, we describe the identification of the tomato (Solanum lycopersicum) PEPR1/2 ORTHOLOG RECEPTOR-LIKE KINASE1 (PORK1) as the TOMATO PROTEIN KINASE1b (TPK1b) interacting protein and demonstrate its biological functions in systemin signaling and tomato immune responses. Tomato PORK1 RNA interference (RNAi) plants with significantly reduced PORK1 expression showed increased susceptibility to tobacco hornworm (Manduca sexta), reduced seedling growth sensitivity to the systemin peptide, and compromised systemin-mediated resistance to Botrytis cinerea. Systemin-induced expression of Proteinase Inhibitor II (PI-II), a classical marker for systemin signaling, was abrogated in PORK1 RNAi plants. Similarly, in response to systemin and wounding, the expression of jasmonate pathway genes was attenuated in PORK1 RNAi plants. TPK1b, a key regulator of tomato defense against B. cinerea and M. sexta, was phosphorylated by PORK1. Interestingly, wounding- and systemin-induced phosphorylation of TPK1b was attenuated when PORK1 expression was suppressed. Our data suggest that resistance to B. cinerea and M. sexta is dependent on PORK1-mediated responses to systemin and subsequent phosphorylation of TPK1b. Altogether, PORK1 regulates tomato systemin, wounding, and immune responses.
© 2018 American Society of Plant Biologists. All rights reserved.
Figures




References
-
- Bar M., Sharfman M., Ron M., Avni A. (2010). BAK1 is required for the attenuation of ethylene-inducing xylanase (Eix)-induced defense responses by the decoy receptor LeEix1. Plant J. 63: 791–800. - PubMed
-
- Beloshistov R.E., et al. (2018). Phytaspase-mediated precursor processing and maturation of the wound hormone systemin. New Phytol. 218: 1167–1178. - PubMed
-
- Bergey D.R., Ryan C.A. (1999). Wound- and systemin-inducible calmodulin gene expression in tomato leaves. Plant Mol. Biol. 40: 815–823. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

