A low-cost paper-based synthetic biology platform for analyzing gut microbiota and host biomarkers
- PMID: 30131493
- PMCID: PMC6104080
- DOI: 10.1038/s41467-018-05864-4
A low-cost paper-based synthetic biology platform for analyzing gut microbiota and host biomarkers
Abstract
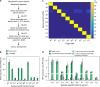
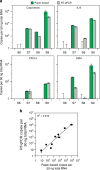
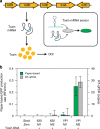
There is a need for large-scale, longitudinal studies to determine the mechanisms by which the gut microbiome and its interactions with the host affect human health and disease. Current methods for profiling the microbiome typically utilize next-generation sequencing applications that are expensive, slow, and complex. Here, we present a synthetic biology platform for affordable, on-demand, and simple analysis of microbiome samples using RNA toehold switch sensors in paper-based, cell-free reactions. We demonstrate species-specific detection of mRNAs from 10 different bacteria that affect human health and four clinically relevant host biomarkers. We develop a method to quantify mRNA using our toehold sensors and validate our platform on clinical stool samples by comparison to RT-qPCR. We further highlight the potential clinical utility of the platform by showing that it can be used to rapidly and inexpensively detect toxin mRNA in the diagnosis of Clostridium difficile infections.
Conflict of interest statement
J.J.C. is an author on a patent application for the paper-based synthetic gene networks US20160312312A1 and a patent for the RNA toehold switch sensors US9550987B2. The remaining authors declare no competing interests.
Figures



Similar articles
-
Cell-Free Paper-Based Analysis of Gut Microbiota and Host Biomarkers.Methods Mol Biol. 2022;2433:351-374. doi: 10.1007/978-1-0716-1998-8_22. Methods Mol Biol. 2022. PMID: 34985756
-
Effects of intestinal colonization by Clostridium difficile and Staphylococcus aureus on microbiota diversity in healthy individuals in China.BMC Infect Dis. 2018 May 3;18(1):207. doi: 10.1186/s12879-018-3111-z. BMC Infect Dis. 2018. PMID: 29724187 Free PMC article.
-
Rapid change of fecal microbiome and disappearance of Clostridium difficile in a colonized infant after transition from breast milk to cow milk.Microbiome. 2016 Oct 7;4(1):53. doi: 10.1186/s40168-016-0198-6. Microbiome. 2016. PMID: 27717398 Free PMC article.
-
Progress of analytical tools and techniques for human gut microbiome research.J Microbiol. 2018 Oct;56(10):693-705. doi: 10.1007/s12275-018-8238-5. Epub 2018 Sep 28. J Microbiol. 2018. PMID: 30267313 Review.
-
Potential for Monitoring Gut Microbiota for Diagnosing Infections and Graft-versus-Host Disease in Cancer and Stem Cell Transplant Patients.Clin Chem. 2017 Nov;63(11):1685-1694. doi: 10.1373/clinchem.2016.259499. Epub 2017 Jul 18. Clin Chem. 2017. PMID: 28720679 Free PMC article. Review.
Cited by
-
Future trends in synthetic biology in Asia.Adv Genet (Hoboken). 2021 Mar 5;2(1):e10038. doi: 10.1002/ggn2.10038. eCollection 2021 Mar. Adv Genet (Hoboken). 2021. PMID: 36618442 Free PMC article.
-
Cell-Free Synthetic Biology Platform for Engineering Synthetic Biological Circuits and Systems.Methods Protoc. 2019 May 14;2(2):39. doi: 10.3390/mps2020039. Methods Protoc. 2019. PMID: 31164618 Free PMC article. Review.
-
RNA Engineering for Public Health: Innovations in RNA-Based Diagnostics and Therapeutics.Annu Rev Chem Biomol Eng. 2021 Jun 7;12:263-286. doi: 10.1146/annurev-chembioeng-101420-014055. Epub 2021 Apr 26. Annu Rev Chem Biomol Eng. 2021. PMID: 33900805 Free PMC article.
-
Genomics of host-pathogen interactions: challenges and opportunities across ecological and spatiotemporal scales.PeerJ. 2019 Nov 5;7:e8013. doi: 10.7717/peerj.8013. eCollection 2019. PeerJ. 2019. PMID: 31720122 Free PMC article.
-
Optimization of E. Coli Tip-Sonication for High-Yield Cell-Free Extract using Finite Element Modeling.AIChE J. 2021 Oct;67(10):e17389. doi: 10.1002/aic.17389. Epub 2021 Aug 5. AIChE J. 2021. PMID: 35663841 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

