Comprehensive panicle phenotyping reveals that qSrn7/FZP influences higher-order branching
- PMID: 30131566
- PMCID: PMC6104091
- DOI: 10.1038/s41598-018-30395-9
Comprehensive panicle phenotyping reveals that qSrn7/FZP influences higher-order branching
Abstract
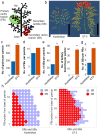
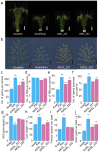
Rice grain number directly affects crop yield. Identifying alleles that improve panicle architecture would greatly aid the development of high-yield varieties. Here, we show that the quantitative trait locus qSrn7 contains rice FRIZZY PANICLE (FZP), a previously reported gene encoding an ERF transcription factor that promotes floral transition. Reduced expression of FZP in the reproductive stage increases the extent of higher order branching of the panicle, resulting in increased grain number. Genotype analysis of this gene in cultivars from the publicly available National Institute of Agrobiological Sciences (NIAS) Core Collection demonstrated that the extent of higher order branching, especially in the upper panicle, was increased in those cultivars carrying the FZP allele associated with qSrn7. Furthermore, chromosome segment substitution lines resulting from a cross between Koshihikari and Kasalath, the latter of which carries qSrn7/FZP, also showed that upper panicle higher order branching and grain yield were increased by qSrn7/FZP. Our findings indicate that qSrn7/FZP influences panicle branching pattern and is thus useful in the breeding of high-yield rice varieties.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

