Androgen-targeted therapy in men with prostate cancer: evolving practice and future considerations
- PMID: 30131604
- PMCID: PMC6370592
- DOI: 10.1038/s41391-018-0079-0
Androgen-targeted therapy in men with prostate cancer: evolving practice and future considerations
Abstract
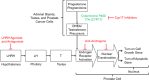
Background: Androgen deprivation therapy (ADT) is foundational in the management of advanced prostate cancer (PCa) and has benefitted from a recent explosion in scientific advances. These include approval of new therapies that suppress testosterone (T) levels or inactivate its function, improvements in diagnostic and assay technologies, identification of lower therapeutic targets for T, discovery of the relevance of germline genetic mutations and identification of the benefits of sequential and combination therapies.
Methods: This review discusses the clinical profiles of the most up-to-date options for ADT, best practices for managing patients with advanced PCa and future directions in therapy.
Results and conclusions: Modern assay technologies reveal that bilateral orchiectomy results in a serum T level of approximately 15 ng/dL as compared to the historical definition of castration of T < 50 ng/dL. Evidence shows that lowering T levels to <20 ng/dL improves patient survival and delays disease progression. Routine monitoring of T in addition to prostate-specific antigen throughout treatment is important to ensure continuing efficacy of T suppression. New drugs that inhibit androgen signaling in combination with traditional ADT suppress T activity to near zero and have significantly improved patient survival. When personalizing ADT regimens physicians should consider a number of factors including initiation and duration of ADT, monitoring of T levels and PSA, the possibility of switching monotherapies if a patient does not achieve adequate T suppression, and consideration of intermittent vs. continuous ADT according to patients' lifestyles, comorbidities, risk factors and tolerance to treatment.
Conflict of interest statement
E.D.C. has held consulting or advisory roles for Bayer, Mdx, Genomic Health, Janssen, Dendreon, Ferring, and Tolmar, and has received grants from NIH and University of Colorado. A.H. has held consulting or advisory roles for Amgen, Astellas, Ferring, Ipsen, Jansen-Cilag, Pfizer, Sanofi, and Takeda. N.L. has held consulting or advisory roles for A&Z, Astellas, Ipsen, Janssen, and Tolmar. K.M. has held consulting or advisory roles for Amgen, Astellas, Bayer, BMS, Ferring, Janssen, Medivation, Merck, MSD, Novartis, Pfizer, Roche, and Tolmar. L.K. has received grants from Abbvie, Ferring, Sanofi, and Tersera. The remaining authors declare that they have no conflicts of interest. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Figures
References
-
- Key statistics for prostate cancer. American Cancer Society; 2016.
-
- Cancer stat facts: common cancer sites. National Cancer Institute Surveillance, Epidemiology, End Results Program. National Institute of Health; 2017.
-
- Cancer stat facts: prostate cancer. National Cancer Institute Surveillance, Epidemiology, and End Results Program. National Institute of Health; 2017.
-
- Wilding G. The importance of steroid hormones in prostate cancer. Cancer Surv. 1992;14:113–130. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous