Electromyography Assessment During Gait in a Robotic Exoskeleton for Acute Stroke
- PMID: 30131756
- PMCID: PMC6090052
- DOI: 10.3389/fneur.2018.00630
Electromyography Assessment During Gait in a Robotic Exoskeleton for Acute Stroke
Abstract
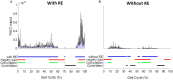
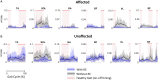
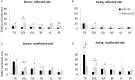
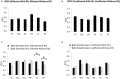
Background: Robotic exoskeleton (RE) based gait training involves repetitive task-oriented movements and weight shifts to promote functional recovery. To effectively understand the neuromuscular alterations occurring due to hemiplegia as well as due to the utilization of RE in acute stroke, there is a need for electromyography (EMG) techniques that not only quantify the intensity of muscle activations but also quantify and compare activation timings in different gait training environments. Purpose: To examine the applicability of a novel EMG analysis technique, Burst Duration Similarity Index (BDSI) during a single session of inpatient gait training in RE and during traditional overground gait training for individuals with acute stroke. Methods: Surface EMG was collected bilaterally with and without the RE device for five participants with acute stroke during the normalized gait cycle to measure lower limb muscle activations. EMG outcomes included integrated EMG (iEMG) calculated from the root-mean-square profiles, and a novel measure, BDSI derived from activation timing comparisons. Results: EMG data demonstrated volitional although varied levels of muscle activations on the affected and unaffected limbs, during gait with and without the RE. During the stance phase mean iEMG of the soleus (p = 0.019) and rectus femoris (RF) (p = 0.017) on the affected side significantly decreased with RE, as compared to without the RE. The differences in mean BDSI scores on the affected side with RE were significantly higher than without RE for the vastus lateralis (VL) (p = 0.010) and RF (p = 0.019). Conclusions: A traditional amplitude analysis (iEMG) and a novel timing analysis (BDSI) techniques were presented to assess the neuromuscular adaptations resulting in lower extremities muscles during RE assisted hemiplegic gait post acute stroke. The RE gait training environment allowed participants with hemiplegia post acute stroke to preserve their volitional neuromuscular activations during gait iEMG and BDSI analyses showed that the neuromuscular changes occurring in the RE environment were characterized by correctly timed amplitude and temporal adaptations. As a result of these adaptations, VL and RF on the affected side closely matched the activation patterns of healthy gait. Preliminary EMG data suggests that the RE provides an effective gait training environment for in acute stroke rehabilitation.
Keywords: electromyography; hemiplegic gait; rehabilitation; robotic exoskeleton; stroke.
Figures

References
-
- Esquenazi A, Maler IC, Schuler TA, Beer SM, Borggraefe I, Campen K, et al. Clinical application of robotics and technology in the restoration of walking. In: Reinkensmeyer D, Dietz V, editors. Neurorehabilitation Technology. Cham: Springer; (2016). p. 223–48.
-
- Byl NN, Abrams GM, Pitsch E, Fedulow I, Kim H, Simkins M, et al. . Chronic stroke survivors achieve comparable outcomes following virtual task specific repetitive training guided by a wearable robotic orthosis (UL-EXO7) and actual task specific repetitive training guided by a physical therapist. J Hand Ther. (2013) 26:343–52; quiz 352. 10.1016/j.jht.2013.06.001 - DOI - PubMed
-
- Morone G, Fusco A, Di Capua P, Coiro P, Pratesi L. Walking training with foot drop stimulator controlled by a tilt sensor to improve walking outcomes: a randomized controlled pilot study in patients with stroke in subacute phase. Stroke Res Treat. (2012) 2012:523564. 10.1155/2012/523564 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

