The Cranberry Extract Oximacro® Exerts in vitro Virucidal Activity Against Influenza Virus by Interfering With Hemagglutinin
- PMID: 30131793
- PMCID: PMC6090095
- DOI: 10.3389/fmicb.2018.01826
The Cranberry Extract Oximacro® Exerts in vitro Virucidal Activity Against Influenza Virus by Interfering With Hemagglutinin
Abstract
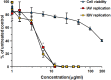
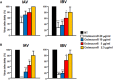
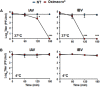
The defense against influenza virus (IV) infections still poses a series of challenges. The current antiviral arsenal against influenza viruses is in fact limited; therefore, the development of new anti-influenza strategies effective against antigenically different viruses is an urgent priority. Bioactive compounds derived from medicinal plants and fruits may provide a natural source of candidates for such broad-spectrum antivirals. In this regard, cranberry (Vaccinium macrocarpon Aiton) extracts on the basis of their recognized anti-adhesive activities against bacteria, may provide potential compounds able to prevent viral attachment to target cells. Nevertheless, only few studies have so far investigated the possible use of cranberry extracts as an antiviral tool. This study focuses on the suitability of a cranberry extract as a direct-acting anti-influenza compound. We show that the novel cranberry extract Oximacro® inhibits influenza A and B viruses (IAV, IBV) replication in vitro because of its high content of A-type proanthocyanidins (PAC-A) dimers and trimers. Mechanistic studies revealed that Oximacro® prevents attachment and entry of IAV and IBV into target cells and exerts a virucidal activity. Oximacro® was observed to interact with the ectodomain of viral hemagglutinin (HA) glycoprotein, thus suggesting the interference with HA functions and a consequent loss of infectivity of IV particles. Fluorescence spectroscopy revealed a reduction in the intrinsic fluorescence of HA protein after incubation with purified dimeric PAC-A (PAC-A2), thus confirming a direct interaction between HA and Oximacro® PAC-A2. In silico docking simulations further supported the in vitro results and indicated that among the different components of the Oximacro® chemical profile, PAC-A2 exhibited the best binding propensity with an affinity below 10 nM. The role of PAC-A2 in the anti-IV activity of Oximacro® was eventually confirmed by the observation that it prevented IAV and IVB replication and caused the loss of infectivity of IV particles, thus indicating PAC-A2 as the major active component of Oximacro®. As a whole, these results suggest Oximacro® as a potential candidate to create novel antiviral agents of natural origin for the prevention of IV infections.
Keywords: Oximacro®; PAC-A2; antiviral and virucidal activities; cranberry extract; dimeric A-type proanthocyanidins; hemagglutinin; influenza virus.
Figures





Similar articles
-
Inhibition of herpes simplex type 1 and type 2 infections by Oximacro(®), a cranberry extract with a high content of A-type proanthocyanidins (PACs-A).Antiviral Res. 2016 Aug;132:154-64. doi: 10.1016/j.antiviral.2016.06.006. Epub 2016 Jun 16. Antiviral Res. 2016. PMID: 27321663
-
Prevention of Urinary Tract Infection with Oximacro, A Cranberry Extract with a High Content of A-Type Proanthocyanidins: A Pre-Clinical Double-Blind Controlled Study.Urol J. 2016 Apr 16;13(2):2640-9. Urol J. 2016. PMID: 27085566 Clinical Trial.
-
Cranberry (Vaccinium macrocarpon) Extract Impairs Nairovirus Infection by Inhibiting the Attachment to Target Cells.Pathogens. 2021 Aug 13;10(8):1025. doi: 10.3390/pathogens10081025. Pathogens. 2021. PMID: 34451488 Free PMC article.
-
Tackling the Future Pandemics: Broad-Spectrum Antiviral Agents (BSAAs) Based on A-Type Proanthocyanidins.Molecules. 2022 Nov 30;27(23):8353. doi: 10.3390/molecules27238353. Molecules. 2022. PMID: 36500445 Free PMC article. Review.
-
Antioxidant and antimicrobial properties of bioactive phytochemicals from cranberry.Postepy Hig Med Dosw (Online). 2016 Dec 31;70(0):1460-1468. doi: 10.5604/17322693.1227896. Postepy Hig Med Dosw (Online). 2016. PMID: 28100853 Review.
Cited by
-
Patient Nutrition and Probiotic Therapy in COVID-19: What Do We Know in 2021?Nutrients. 2021 Sep 26;13(10):3385. doi: 10.3390/nu13103385. Nutrients. 2021. PMID: 34684384 Free PMC article. Review.
-
Marine Fungi from the Sponge Grantia compressa: Biodiversity, Chemodiversity, and Biotechnological Potential.Mar Drugs. 2019 Apr 11;17(4):220. doi: 10.3390/md17040220. Mar Drugs. 2019. PMID: 30978942 Free PMC article.
-
Novel and Alternative Therapeutic Strategies for Controlling Avian Viral Infectious Diseases: Focus on Infectious Bronchitis and Avian Influenza.Front Vet Sci. 2022 Jul 22;9:933274. doi: 10.3389/fvets.2022.933274. eCollection 2022. Front Vet Sci. 2022. PMID: 35937298 Free PMC article. Review.
-
Antiviral Effect of Isoquercitrin against Influenza A Viral Infection via Modulating Hemagglutinin and Neuraminidase.Int J Mol Sci. 2022 Oct 28;23(21):13112. doi: 10.3390/ijms232113112. Int J Mol Sci. 2022. PMID: 36361900 Free PMC article.
-
Strawberry and Ginger Silver Nanoparticles as Potential Inhibitors for SARS-CoV-2 Assisted by In Silico Modeling and Metabolic Profiling.Antibiotics (Basel). 2021 Jul 6;10(7):824. doi: 10.3390/antibiotics10070824. Antibiotics (Basel). 2021. PMID: 34356745 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

