Production, quality control, stability, and potency of cGMP-produced Plasmodium falciparum RH5.1 protein vaccine expressed in Drosophila S2 cells
- PMID: 30131879
- PMCID: PMC6098134
- DOI: 10.1038/s41541-018-0071-7
Production, quality control, stability, and potency of cGMP-produced Plasmodium falciparum RH5.1 protein vaccine expressed in Drosophila S2 cells
Abstract
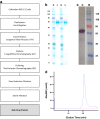
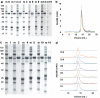
Plasmodium falciparum reticulocyte-binding protein homolog 5 (PfRH5) is a leading asexual blood-stage vaccine candidate for malaria. In preparation for clinical trials, a full-length PfRH5 protein vaccine called "RH5.1" was produced as a soluble product under cGMP using the ExpreS2 platform (based on a Drosophila melanogaster S2 stable cell line system). Following development of a high-producing monoclonal S2 cell line, a master cell bank was produced prior to the cGMP campaign. Culture supernatants were processed using C-tag affinity chromatography followed by size exclusion chromatography and virus-reduction filtration. The overall process yielded >400 mg highly pure RH5.1 protein. QC testing showed the MCB and the RH5.1 product met all specified acceptance criteria including those for sterility, purity, and identity. The RH5.1 vaccine product was stored at -80 °C and is stable for over 18 months. Characterization of the protein following formulation in the adjuvant system AS01B showed that RH5.1 is stable in the timeframe needed for clinical vaccine administration, and that there was no discernible impact on the liposomal formulation of AS01B following addition of RH5.1. Subsequent immunization of mice confirmed the RH5.1/AS01B vaccine was immunogenic and could induce functional growth inhibitory antibodies against blood-stage P. falciparum in vitro. The RH5.1/AS01B was judged suitable for use in humans and has since progressed to phase I/IIa clinical trial. Our data support the future use of the Drosophila S2 cell and C-tag platform technologies to enable cGMP-compliant biomanufacture of other novel and "difficult-to-express" recombinant protein-based vaccines.
Conflict of interest statement
A.D.D., M.K.H., and S.J.D. are named inventors on patent applications relating to PfRH5 and/or other malaria vaccines. M.S., L.P., T.J., and W.A.d.J. are employees of and W.A.d.J. is a shareholder in ExpreS2ion Biotechnologies, which has developed and is marketing the ExpreS2 cell expression platform. F.J.D. is an employee of Thermo Fisher Scientific who is the commercial provider of CaptureSelect™ C-tag products. The remaining authors declare no competing interests.
Figures




References
-
- WHO. World Malaria Report. (2015).
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

