Endothelial Cell Metabolism in Atherosclerosis
- PMID: 30131957
- PMCID: PMC6090045
- DOI: 10.3389/fcell.2018.00082
Endothelial Cell Metabolism in Atherosclerosis
Abstract
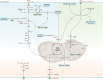
Atherosclerosis and its sequelae, such as myocardial infarction and stroke, are the leading cause of death worldwide. Vascular endothelial cells (EC) play a critical role in vascular homeostasis and disease. Atherosclerosis as well as its independent risk factors including diabetes, obesity, and aging, are hallmarked by endothelial activation and dysfunction. Metabolic pathways have emerged as key regulators of many EC functions, including angiogenesis, inflammation, and barrier function, processes which are deregulated during atherogenesis. In this review, we highlight the role of glucose, fatty acid, and amino acid metabolism in EC functions during physiological and pathological states, specifically atherosclerosis, diabetes, obesity and aging.
Keywords: aging; atherosclerosis; endothelial cells; hyperglycemia; hyperlipidemia; inflammation; metabolism.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

