Efficient Nose-to-Lung Aerosol Delivery with an Inline DPI Requiring Low Actuation Air Volume
- PMID: 30132207
- PMCID: PMC7253151
- DOI: 10.1007/s11095-018-2473-7
Efficient Nose-to-Lung Aerosol Delivery with an Inline DPI Requiring Low Actuation Air Volume
Abstract
Purpose: To demonstrate efficient aerosol delivery through an in vitro nasal model using a dry powder inhaler (DPI) requiring low actuation air volumes (LV) applied during low-flow nasal cannula (LFNC) therapy.
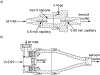
Methods: A previously developed LV-DPI was connected to a LFNC system with 4 mm diameter tubing. System connections and the nasal cannula interface were replaced with streamlined components. To simulate nasal respiration, an in vitro nasal model was connected to a downstream lung simulator that produced either passive or deep nasal respiration. Performance of a commercial mesh nebulizer system was also considered.
Results: For the optimized system, steady state cannula emitted dose was 75% of the capsule loaded dose. With cyclic nasal breathing, delivery efficiency to the tracheal filter was 53-55% of the loaded dose, which was just under the design target of 60%. Compared with a commercially available mesh nebulizer, the optimal LV-DPI was 40-fold more efficient and 150 times faster in terms of delivering aerosol to the lungs.
Conclusions: The optimized LV-DPI system is capable of high efficiency lung delivery of powder aerosols through a challenging nasal cannula interface.
Keywords: Dry powder inhaler (DPI); inline DPI; low flow nasal cannula; low flow oxygen; nasal cannula aerosol; pharmaceutical aerosol.
Figures





References
-
- Pornputtapitak W, El-Gendy N, Mermis J, O’Brein-Ladner A, and Berkland C. NanoCluster budesondie formulations enable efficient drug delivery driven by mechanical ventilation. International Journal of Pharmaceutics. 2014;462:19–28. - PubMed
-
- Tang P, Chan HK, Rajbhandari D, and Phipps P. Method to Introduce Mannitol Powder to Intubated Patients to Improve Sputum Clearance. Journal Of Aerosol Medicine And Pulmonary Drug Delivery. 2011;24:1–9. - PubMed
-
- Laube BL, Sharpless G, Shermer C, Sullivan V, and Powell K. Deposition of dry powder generated by solovent in Sophia Anatomical infant nose-throat (SAINT) model. Aerosol Science and Technology. 2012;46:514–520.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

