Expanding the Boundaries of Biotherapeutics with Bispecific Antibodies
- PMID: 30132211
- PMCID: PMC6182456
- DOI: 10.1007/s40259-018-0299-9
Expanding the Boundaries of Biotherapeutics with Bispecific Antibodies
Abstract
Bispecific antibodies have moved from being an academic curiosity with therapeutic promise to reality, with two molecules being currently commercialized (Hemlibra® and Blincyto®) and many more in clinical trials. The success of bispecific antibodies is mainly due to the continuously growing number of mechanisms of actions (MOA) they enable that are not accessible to monoclonal antibodies. One of the earliest MOA of bispecific antibodies and currently the one with the largest number of clinical trials is the redirecting of the cytotoxic activity of T-cells for oncology applications, now extending its use in infective diseases. The use of bispecific antibodies for crossing the blood-brain barrier is another important application because of its potential to advance the therapeutic options for neurological diseases. Another noteworthy application due to its growing trend is enabling a more tissue-specific delivery or activity of antibodies. The different molecular solutions to the initial hurdles that limited the development of bispecific antibodies have led to the current diverse set of bispecific or multispecific antibody formats that can be grouped into three main categories: IgG-like formats, antibody fragment-based formats, or appended IgG formats. The expanded applications of bispecific antibodies come at the price of additional challenges for clinical development. The rising complexity in their structure may increase the risk of immunogenicity and the multiple antigen specificity complicates the selection of relevant species for safety assessment.
Conflict of interest statement
Diego Ellerman and Bushra Husain are full time employees of Genentech Inc, and own stocks in Roche. Diego Ellerman has two patent applications related to the use of bispecific antibodies.
Figures

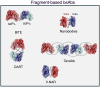

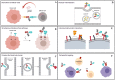
Similar articles
-
"BIClonals": Production of Bispecific Antibodies in IgG Format in Transiently Transfected Mammalian Cells.Methods Mol Biol. 2019;1904:431-454. doi: 10.1007/978-1-4939-8958-4_22. Methods Mol Biol. 2019. PMID: 30539485
-
Generation of dual-variable-domain immunoglobulin molecules for dual-specific targeting.Methods Enzymol. 2012;502:25-41. doi: 10.1016/B978-0-12-416039-2.00002-1. Methods Enzymol. 2012. PMID: 22208980
-
A novel glycoengineered bispecific antibody format for targeted inhibition of epidermal growth factor receptor (EGFR) and insulin-like growth factor receptor type I (IGF-1R) demonstrating unique molecular properties.J Biol Chem. 2014 Jul 4;289(27):18693-706. doi: 10.1074/jbc.M113.528109. Epub 2014 May 19. J Biol Chem. 2014. PMID: 24841203 Free PMC article.
-
The use of CrossMAb technology for the generation of bi- and multispecific antibodies.MAbs. 2016 Aug-Sep;8(6):1010-20. doi: 10.1080/19420862.2016.1197457. Epub 2016 Jun 10. MAbs. 2016. PMID: 27285945 Free PMC article. Review.
-
Applications and challenges in designing VHH-based bispecific antibodies: leveraging machine learning solutions.MAbs. 2024 Jan-Dec;16(1):2341443. doi: 10.1080/19420862.2024.2341443. Epub 2024 Apr 26. MAbs. 2024. PMID: 38666503 Free PMC article. Review.
Cited by
-
New immune cell engagers for cancer immunotherapy.Nat Rev Immunol. 2024 Jul;24(7):471-486. doi: 10.1038/s41577-023-00982-7. Epub 2024 Jan 25. Nat Rev Immunol. 2024. PMID: 38273127 Review.
-
End-To-End Automated Intact Protein Mass Spectrometry for High-Throughput Screening and Characterization of Bispecific and Multispecific Antibodies.Anal Chem. 2024 Nov 12;96(45):18287-18300. doi: 10.1021/acs.analchem.4c04833. Epub 2024 Oct 31. Anal Chem. 2024. PMID: 39479787
-
An in silico Model of T Cell Infiltration Dynamics Based on an Advanced in vitro System to Enhance Preclinical Decision Making in Cancer Immunotherapy.Front Pharmacol. 2022 May 2;13:837261. doi: 10.3389/fphar.2022.837261. eCollection 2022. Front Pharmacol. 2022. PMID: 35586042 Free PMC article.
-
Monoclonal Antibodies as Neurological Therapeutics.Pharmaceuticals (Basel). 2021 Jan 26;14(2):92. doi: 10.3390/ph14020092. Pharmaceuticals (Basel). 2021. PMID: 33530460 Free PMC article. Review.
-
Discovery of amivantamab (JNJ-61186372), a bispecific antibody targeting EGFR and MET.J Biol Chem. 2021 Jan-Jun;296:100641. doi: 10.1016/j.jbc.2021.100641. Epub 2021 Apr 8. J Biol Chem. 2021. PMID: 33839159 Free PMC article.
References
-
- Mahmuda A, et al. Monoclonal antibodies: a review of therapeutic applications and future prospects. Trop J Pharm Res. 2017 doi: 10.4314/tjpr.v16i3.29. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

