Immune-Related Thyroiditis with Immune Checkpoint Inhibitors
- PMID: 30132401
- PMCID: PMC6157359
- DOI: 10.1089/thy.2018.0116
Immune-Related Thyroiditis with Immune Checkpoint Inhibitors
Abstract
Background: Although immune-related thyroiditis (irT) with immune checkpoint inhibitors (ICI) is a common consequence, its natural course and management recommendations are not well characterized in existing guidelines. This study sought to investigate the evolution of irT and describe its course and sequelae.
Methods: This was a retrospective study of cancer patients treated with ICI between November 2014 and July 2016 at MD Anderson Cancer Center and referred for endocrinology evaluation for suspected irT. Patients included had normal baseline thyroid function tests prior to starting ICI and developed thyrotoxicosis due to irT.
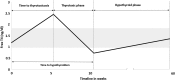
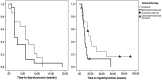
Results: Of 657 patients treated with ICI during the study period, 43(6.5%) met the inclusion criteria. ICI included: ipilimumab + nivolumab (40%), nivolumab (33%), pembrolizumab (21%), and other (7%). Cancer diagnoses observed were melanoma (23%), renal-cell carcinoma (21%), lung cancer (19%), bladder cancer (12%), colon cancer (9%), and other cancers (15%). Median time from ICI start to thyrotoxicosis was 5.3 weeks (range 0.6-19.6 weeks). Clinically, patients presented with painless thyroiditis, and 67% were asymptomatic during the thyrotoxicosis phase. Thyrotoxicosis lasted a median of six weeks (range 2.6-39.7 weeks). Hypothyroidism developed in 37 (84%) patients at a median of 10.4 weeks (range 3.4-48.7 weeks) after starting ICI. These patients remained on levothyroxine and ICI at a median follow-up of 57.4 weeks (range 1-156.7 weeks) from hypothyroidism onset. Four patients recovered without initiating levothyroxine and remained euthyroid at a median follow-up of 11.35 months (range 4.43-14.43 months). Subgroup analysis of ipilimumab + nivolumab versus nivolumab alone showed a median time to thyrotoxicosis of two weeks [confidence interval (CI) 3.5-8.4] versus six weeks ([CI 1.2-2.8]; p = 0.26) and time to hypothyroidism of 10 weeks [CI 8.1-11.9] versus 17 weeks ([CI 8.8-25.2]; p = 0.029) after starting ICI. Thyroid peroxidase and thyroglobulin antibodies were present in 45% and 33% at the time of irT diagnosis.
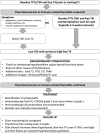
Conclusions: IrT manifests as an early onset of thyrotoxicosis, which is largely asymptomatic, followed by rapid transition to hypothyroidism requiring long-term levothyroxine substitution. The evolution of irT is more rapid with combination ICI. Frequent monitoring of thyroid function tests during ICI is warranted. Future guidelines need to recognize this entity and incorporate their management.
Keywords: immunotherapy; nivolumab; pembrolizumab; side effects; thyroiditis.
Conflict of interest statement
R.D. is a member of the Bristol Myers Squibb advisory board. The remaining authors have nothing to disclose.
Figures
References
-
- Walker LS, Sansom DM. 2011. The emerging role of CTLA4 as a cell-extrinsic regulator of T cell responses. Nat Rev Immunol 11:852–863 - PubMed
-
- Fife BT, Bluestone JA. 2008. Control of peripheral T-cell tolerance and autoimmunity via the CTLA-4 and PD-1 pathways. Immunol Rev 224:166–182 - PubMed
-
- Dadu R, Zobniw C, Diab A. 2016. Managing adverse events with immune checkpoint agents. Cancer J 22:121–129 - PubMed
-
- Boutros C, Tarhini A, Routier E, Lambotte O, Ladurie FL, Carbonnel F, Izzeddine H, Marabelle A, Champiat S, Berdelou A, Lanoy E, Texier M, Libenciuc C, Eggermont AM, Soria JC, Mateus C, Robert C. 2016. Safety profiles of anti-CTLA-4 and anti-PD-1 antibodies alone and in combination. Nat Rev Clin Oncol 13:473–486 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

