Specialized mechanoreceptor systems in rodent glabrous skin
- PMID: 30132906
- PMCID: PMC6187043
- DOI: 10.1113/JP276608
Specialized mechanoreceptor systems in rodent glabrous skin
Abstract
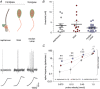
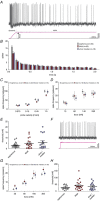
Key points: An ex vivo preparation was developed to record from single sensory fibres innervating the glabrous skin of the mouse forepaw. The density of mechanoreceptor innervation of the forepaw glabrous skin was found to be three times higher than that of hindpaw glabrous skin. Rapidly adapting mechanoreceptors that innervate Meissner's corpuscles were severalfold more responsive to slowly moving stimuli in the forepaw compared to those innervating hindpaw skin. We found a distinct group of small hairs in the centre of the mouse hindpaw glabrous skin that were exclusively innervated by directionally sensitive D-hair receptors. The directional sensitivity, but not the end-organ anatomy, were the opposite to D-hair receptors in the hairy skin. Glabrous skin hairs in the hindpaw are not ubiquitous in rodents, but occur in African and North American species that diverged more than 65 million years ago.
Abstract: Rodents use their forepaws to actively interact with their tactile environment. Studies on the physiology and anatomy of glabrous skin that makes up the majority of the forepaw are almost non-existent in the mouse. Here we developed a preparation to record from single sensory fibres of the forepaw and compared anatomical and physiological receptor properties to those of the hindpaw glabrous and hairy skin. We found that the mouse forepaw skin is equipped with a very high density of mechanoreceptors; >3 times more than hindpaw glabrous skin. In addition, rapidly adapting mechanoreceptors that innervate Meissner's corpuscles of the forepaw were severalfold more sensitive to slowly moving mechanical stimuli compared to their counterparts in the hindpaw glabrous skin. All other mechanoreceptor types as well as myelinated nociceptors had physiological properties that were invariant regardless of which skin area they occupied. We discovered a novel D-hair receptor innervating a small group of hairs in the middle of the hindpaw glabrous skin in mice. These glabrous skin D-hair receptors were direction sensitive albeit with an orientation sensitivity opposite to that described for hairy skin D-hair receptors. Glabrous skin hairs do not occur in all rodents, but are present in North American and African rodent species that diverged more than 65 million years ago. The function of these specialized hairs is unknown, but they are nevertheless evolutionarily very ancient. Our study reveals novel physiological specializations of mechanoreceptors in the glabrous skin that likely evolved to facilitate tactile exploration.
Keywords: evolution; mechanoreceptor; touch sensation.
© 2018 The Authors The Journal of Physiology published by John Wiley & Sons Ltd on behalf of The Physiological Society.
Figures




Similar articles
-
Sensory innervation of the raccoon forepaw: 1. Receptor types in glabrous and hairy skin and deep tissue.Somatosens Res. 1986;4(1):43-62. doi: 10.3109/07367228609144597. Somatosens Res. 1986. PMID: 3797914
-
Evidence for sparse C-tactile afferent innervation of glabrous human hand skin.J Neurophysiol. 2021 Jan 1;125(1):232-237. doi: 10.1152/jn.00587.2020. Epub 2020 Dec 9. J Neurophysiol. 2021. PMID: 33296618
-
Skin-type-dependent development of murine mechanosensory neurons.Dev Cell. 2023 Oct 23;58(20):2032-2047.e6. doi: 10.1016/j.devcel.2023.07.020. Epub 2023 Aug 21. Dev Cell. 2023. PMID: 37607547 Free PMC article.
-
Mechanosensory perception: are there contributions from bone-associated receptors?Clin Exp Pharmacol Physiol. 2005 Jan-Feb;32(1-2):100-8. doi: 10.1111/j.1440-1681.2005.04136.x. Clin Exp Pharmacol Physiol. 2005. PMID: 15730443 Review.
-
Hairy sensation.Physiology (Bethesda). 2013 May;28(3):142-50. doi: 10.1152/physiol.00059.2012. Physiology (Bethesda). 2013. PMID: 23636260 Review.
Cited by
-
Sex-Dependent Reduction in Mechanical Allodynia in the Sural-Sparing Nerve Injury Model in Mice Lacking Merkel Cells.J Neurosci. 2021 Jun 30;41(26):5595-5619. doi: 10.1523/JNEUROSCI.1668-20.2021. Epub 2021 May 24. J Neurosci. 2021. PMID: 34031166 Free PMC article.
-
Piezo2 voltage-block regulates mechanical pain sensitivity.Brain. 2024 Oct 3;147(10):3487-3500. doi: 10.1093/brain/awae227. Brain. 2024. PMID: 38984717 Free PMC article.
-
Somatosensory corticospinal tract axons sprout within the cervical cord following a dorsal root/dorsal column spinal injury in the rat.J Comp Neurol. 2020 Jun;528(8):1293-1306. doi: 10.1002/cne.24826. Epub 2019 Dec 9. J Comp Neurol. 2020. PMID: 31769033 Free PMC article.
-
A novel task to investigate vibrotactile detection in mice.PLoS One. 2023 Apr 20;18(4):e0284735. doi: 10.1371/journal.pone.0284735. eCollection 2023. PLoS One. 2023. PMID: 37079581 Free PMC article.
-
In vivo calcium imaging reveals directional sensitivity of C-low threshold mechanoreceptors.J Physiol. 2025 Feb;603(4):895-908. doi: 10.1113/JP286631. Epub 2025 Jan 15. J Physiol. 2025. PMID: 39810695 Free PMC article.
References
-
- Adriaensen H, Gybels J, Handwerker HO & Van Hees J (1983). Response properties of thin myelinated (Aδ) fibers in human skin nerves. J Neurophysiol 49, 111–122. - PubMed
-
- Arcourt A, Gorham L, Dhandapani R, Prato V, Taberner FJ, Wende H, Gangadharan V, Birchmeier C, Heppenstall PA & Lechner SG (2017). Touch receptor‐derived sensory information alleviates acute pain signaling and fine‐tunes nociceptive reflex coordination. Neuron 93, 179–193. - PubMed
-
- Bennett NC & Faulkes CG (2000). African Mole Rats: Ecology and Eusociality. Cambridge University Press, Cambridge.
-
- Bennett NC & Jarvis JUM (1988). The social structure and reproductive biology of colonies of the mole‐rat, Cryptomys damarensis (Rodentia, Bathyergidae). J Mammal 69, 293–302.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

