Role of retinal pigment epithelium-derived exosomes and autophagy in new blood vessel formation
- PMID: 30133118
- PMCID: PMC6201377
- DOI: 10.1111/jcmm.13730
Role of retinal pigment epithelium-derived exosomes and autophagy in new blood vessel formation
Abstract
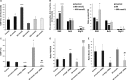
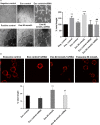
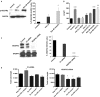
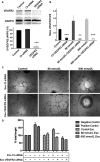
Autophagy and exosome secretion play important roles in a variety of physiological and disease states, including the development of age-related macular degeneration. Previous studies have demonstrated that these cellular mechanisms share common pathways of activation. Low oxidative damage in ARPE-19 cells, alters both autophagy and exosome biogenesis. Moreover, oxidative stress modifies the protein and genetic cargo of exosomes, possibly affecting the fate of surrounding cells. In order to understand the connection between these two mechanisms and their impact on angiogenesis, stressed ARPE-19 cells were treated with a siRNA-targeting Atg7, a key protein for the formation of autophagosomes. Subsequently, we observed the formation of multivesicular bodies and the release of exosomes. Released exosomes contained VEGFR2 as part of their cargo. This receptor for VEGF-which is critical for the development of new blood vessels-was higher in exosome populations released from stressed ARPE-19. While stressed exosomes enhanced tube formation, exosomes became ineffective after silencing VEGFR2 in ARPE-19 cells and were, consequently, unable to influence angiogenesis. Moreover, vessel sprouting in the presence of stressed exosomes seems to follow a VEGF-independent pathway. We propose that abnormal vessel growth correlates with VEGFR2-expressing exosomes release from stressed ARPE-19 cells, and is directly linked to autophagy.
Keywords: VEGFR2; angiogenesis; autophagy; exosomes; oxidative stress; retina; retinal pigment epithelium; siRNA.
© 2018 The Authors. Journal of Cellular and Molecular Medicine published by John Wiley & Sons Ltd and Foundation for Cellular and Molecular Medicine.
Figures


References
-
- Bonet‐Ponce L, Saez‐Atienzar S, da Casa C, et al. On the mechanism underlying ethanol‐induced mitochondrial dynamic disruption and autophagy response. Biochim Biophys Acta. 2015;1852:1400‐1409. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

