Hierarchical Assemblies of Supramolecular Coordination Complexes
- PMID: 30133252
- PMCID: PMC6348110
- DOI: 10.1021/acs.accounts.8b00233
Hierarchical Assemblies of Supramolecular Coordination Complexes
Abstract
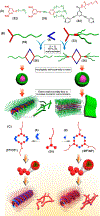
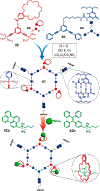
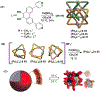
Hierarchical self-assembly (HAS) is a multilevel organization process that first assembles elementary molecular units into ordered secondary structures via noncovalent interactions, which further act as the building blocks to form more complex multifunctional superstructures at the next level(s). The HAS strategy has been used as a versatile method for the preparation of soft-matter nanoarchitectures of defined size and morphologies, tunable luminescence, and biological importance. However, such preparation can be greatly simplified if well-defined dynamic structures are employed as the cores that upon linking form the desired nanoarchitectures. Discrete supramolecular coordination complexes (SCCs) with well-defined shapes, sizes, and internal cavities have been widely employed to construct hierarchical systems with functional diversity. This Account summarizes the prevailing strategies used in recent years in the preparation of SCC-based HASs and illustrates how the combination of dynamic metal-ligand coordination with other interactions was used to obtain hierarchical systems with interesting properties. HASs with dual orthogonal interactions involving coordination-driven self-assembly and hydrogen bonding/host-guest interaction generally result in robust and flexible supramolecular gels. Likewise, hybridization of SCCs with a suitable dynamic covalent network via a hierarchical strategy is useful to prepare materials with self-healing properties. The intrinsic positive charges of the SCCs also make them suitable precursors for the construction of HASs via electrostatic interactions with negatively charged biological/abiological molecules. Furthermore, the interplay between the hydrophilic and lipophilic characters of HASs by varying the number and spacial orientation of alkyl/oxyethylene chains of the SCC is a simple yet controllable approach to prepare ordered and tunable nanostructures. Certain SCC-cored hierarchical systems exhibit reversible polymorphism, typically between micellar, nanofiber, and vesicular phases, in response to various external perturbations: heat, photoirradiation, pH-variance, redox-active agents, etc. At the same time, multiple noncovalent interaction mediated HASs are growing in numbers and are promising candidates for obtaining functionally diverse materials. The photophysical properties of SCC-based HASs have been used in many analytical applications. For example, embedding tetraphenylethene (TPE)-based pyridyl ligands within metallo-supramolecular structures partially restricts the molecular rotations of its phenyl rings, endowing the resultant SCCs with weak emissions. Further aggregation of such HASs in suitable solvents results in a marked enhancement in emission intensity along with quantum yields. They act as sensitive sensors for different analytes, including pathogens, drugs, etc. HASs are also useful to develop multidrug systems with cooperative chemotherapeutic effects. Hence, the use of HASs with theranostic SCCs combining cell-imaging agents and chemotherapeutic scaffolds is a promising drug delivery strategy for cancer theranostics. At the same time, their responsiveness to stimuli, oftentimes due to the dynamic nature of the metal-ligand interactions, play an important role in drug release via a disassembly mechanism.
Conflict of interest statement
Notes
The authors declare no competing financial interest.
Figures








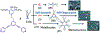









References
-
- Chen L-J; Yang H-B; Shionoya M Chiral Metallosupramolecular Architectures. Chem. Soc. Rev 2017, 46, 2555–2576. - PubMed
-
- Cook TR; Stang PJ Recent Developments in the Preparation and Chemistry of Metallacycles and Metallacages via Coordination. Chem. Rev 2015, 115, 7001–7045. - PubMed
-
- Lifschitz AM; Rosen MS; McGuirk CM; Mirkin CA Allosteric Supramolecular Coordination Constructs. J. Am. Chem. Soc 2015, 137, 7252–7261. - PubMed
-
- Newkome GR; Moorefield CN From 1 → 3 Dendritic Designs to Fractal Supramacromolecular Constructs: Understanding the Pathway to the Sierpinśki Gasket. Chem. Soc. Rev 2015, 44, 3954–3967. - PubMed
-
- Brown CJ; Toste FD; Bergman RG; Raymond KN Supramolecular Catalysis in Metal–Ligand Cluster Hosts. Chem. Rev 2015, 115, 3012–3035. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

