Development of Locked Nucleic Acid Antisense Oligonucleotides Targeting Ebola Viral Proteins and Host Factor Niemann-Pick C1
- PMID: 30133337
- PMCID: PMC6425983
- DOI: 10.1089/nat.2018.0722
Development of Locked Nucleic Acid Antisense Oligonucleotides Targeting Ebola Viral Proteins and Host Factor Niemann-Pick C1
Abstract
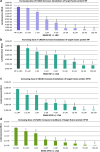
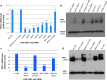
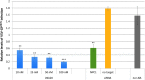
The Ebola virus is a zoonotic pathogen that can cause severe hemorrhagic fever in humans, with up to 90% lethality. The deadly 2014 Ebola outbreak quickly made an unprecedented impact on human lives. While several vaccines and therapeutics are under development, current approaches contain several limitations, such as virus mutational escape, need for formulation or refrigeration, poor scalability, long lead-time, and high cost. To address these challenges, we developed locked nucleic acid (LNA)-modified antisense oligonucleotides (ASOs) to target critical Ebola viral proteins and the human intracellular host protein Niemann-Pick C1 (NPC1), required for viral entry into infected cells. We generated noninfectious viral luciferase reporter assays to identify LNA ASOs that inhibit translation of Ebola viral proteins in vitro and in human cells. We demonstrated specific inhibition of key Ebola genes VP24 and nucleoprotein, which inhibit a proper immune response and promote Ebola virus replication, respectively. We also identified LNA ASOs targeting human host factor NPC1 and demonstrated reduced infection by chimeric vesicular stomatitis virus harboring the Ebola glycoprotein, which directly binds to NPC1 for viral infection. These results support further in vivo testing of LNA ASOs in infectious Ebola virus disease animal models as potential therapeutic modalities for treatment of Ebola.
Keywords: Ebola; Ebola virus (EBOV); Niemann-Pick C1 (NPC1); antisense oligonucleotides; locked nucleic acids.
Conflict of interest statement
No competing financial interests exist.
Figures

References
-
- Moghadam SRJ, Omidi N, Bayrami S, Moghadam SJ. and Seyed Alinaghi S. (2015). Ebola viral disease: a review literature. Asian Pac J Trop Biomed 5:260–267
-
- Clark DV, Kibuuka H, Millard M, Wakabi S, Lukwago L, Taylor A, Eller MA, Eller LA, Michael NL, et al. (2015). Long-term sequelae after Ebola virus disease in Bundibugyo, Uganda: a retrospective cohort study. Lancet Infect Dis 15:905–912 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

