In vivo analysis of outer arm dynein transport reveals cargo-specific intraflagellar transport properties
- PMID: 30133350
- PMCID: PMC6254574
- DOI: 10.1091/mbc.E18-05-0291
In vivo analysis of outer arm dynein transport reveals cargo-specific intraflagellar transport properties
Abstract
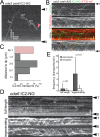
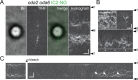
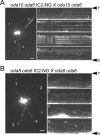
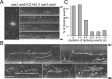
Outer dynein arms (ODAs) are multiprotein complexes that drive flagellar beating. Based on genetic and biochemical analyses, ODAs preassemble in the cell body and then move into the flagellum by intraflagellar transport (IFT). To study ODA transport in vivo, we expressed the essential intermediate chain 2 tagged with mNeonGreen (IC2-NG) to rescue the corresponding Chlamydomonas reinhardtii mutant oda6. IC2-NG moved by IFT; the transport was of low processivity and increased in frequency during flagellar growth. As expected, IFT of IC2-NG was diminished in oda16, lacking an ODA-specific IFT adapter, and in ift46 IFT46ΔN lacking the ODA16-interacting portion of IFT46. IFT loading appears to involve ODA16-dependent recruitment of ODAs to basal bodies followed by handover to IFT. Upon unloading from IFT, ODAs rapidly docked to the axoneme. Transient docking still occurred in the docking complex mutant oda3 indicating that the docking complex stabilizes rather than initiates ODA-microtubule interactions. In full-length flagella, ODAs continued to enter and move inside cilia by short-term bidirectional IFT and diffusion and the newly imported complexes frequently replaced axoneme-bound ODAs. We propose that the low processivity of ODA-IFT contributes to flagellar maintenance by ensuring the availability of replacement ODAs along the length of flagella.
Figures




References
-
- Brokaw CJ. (1994). Control of flagellar bending: a new agenda based on dynein diversity. Cell Motil Cytoskeleton , 199–204. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

