Therapeutic control of leishmaniasis by inhibitors of the mammalian target of rapamycin
- PMID: 30133440
- PMCID: PMC6122837
- DOI: 10.1371/journal.pntd.0006701
Therapeutic control of leishmaniasis by inhibitors of the mammalian target of rapamycin
Abstract
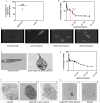
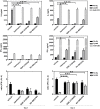
Leishmaniasis is a serious global health problem affecting many people worldwide. While patients with leishmaniasis can be treated with several agents, drug toxicicty and the emergence of resistant strains render available treatments ineffective in the long run. Inhibitors of the mammalian target of rapamycin (mTOR) have been demonstrated to exert anti-pathogen properties. In this study, we tested the therapeutic efficacy of several mTOR inhibitors in controlling infection with Leishmania major. Rapamycin, GSK-2126458 and KU-0063794 were administered to BALB/c mice, which had received an intrafootpad injection of the parasite. Footpad swelling and parasite burden were assessed, and cytokine production by mouse splenocytes and phenotypic changes in draining lymph node cells were evaluated. Treatment with a clinically relevant dose of rapamycin or with GSK-2126458, but not with KU-0063794, dramatically lowered both the footpad swelling and the parasite load in the draining lymph node. Importantly, the employed dose of rapamycin did not kill the promastigotes in vitro as judged by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assays and electron microscopy. Moreover, the IL-4 production capacity of splenocytes harvested from infected mice that were treated with rapamycin was significantly reduced. Consequently, the IFN-γ:IL-4 production ratio was elevated, suggesting a T helper-type 1 (Th1)-skewed cytokine profile. Finally, the expression level of CD69, an early activation marker, on splenic and lymph node CD4+ and CD8+ T cells was enhanced in rapamycin-treated mice. Taken together, our findings suggest that select mTOR inhibitors may be used in therapeutic settings for the management of leishmaniasis. We propose that the beneficial effects of such inhibitors stem from their immunomodulatory properties. Therefore, the adjuvanticity of mTOR inhibitors may also be considered in vaccination strategies against Leishmania species.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



References
-
- Vendrame CM, Souza LD, Carvalho MD, Salgado K, Carvalho EM, et al. (2010) Insulin-like growth factor-I induced and constitutive arginase activity differs among isolates of Leishmania derived from patients with diverse clinical forms of Leishmania braziliensis infection. Transactions of the Royal Society of Tropical Medicine and Hygiene 104: 566–568. 10.1016/j.trstmh.2010.03.007 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

