Treatment of chronic hepatitis B naïve patients with a therapeutic vaccine containing HBs and HBc antigens (a randomized, open and treatment controlled phase III clinical trial)
- PMID: 30133478
- PMCID: PMC6104936
- DOI: 10.1371/journal.pone.0201236
Treatment of chronic hepatitis B naïve patients with a therapeutic vaccine containing HBs and HBc antigens (a randomized, open and treatment controlled phase III clinical trial)
Abstract
Context: Current drugs for chronic hepatitis B therapy have a poor efficacy in terms of post-treatment sustained viral suppression and generate important side effects during and after therapy. Therapeutic vaccination with HBV antigens is an attractive alternative to test.
Objective: Evaluating the efficacy of a therapeutic vaccine candidate (designated NASVAC) containing both hepatitis B surface antigen (HBsAg) and core antigen (HBcAg) versus pegylated interferon (Peg-IFN) in naïve chronic hepatitis B patients.
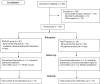
Design, setting, participants: An open phase III, randomised and treatment controlled clinical trial was conducted in a total of 160 CHB patients, allocated into two groups of 80 patients each to receive NASVAC or Peg-IFN. The vaccine formulation comprised 100 μg of each HBsAg and HBcAg, and was administered in 2 cycles of 5 doses. The control group received 48 subcutaneous injections of Peg-IFN alfa 2b, 180 μg per dose, every week, for 48 consecutive weeks.
Main outcome measure: The primary outcome measure was in relation with the proportion of patients showing reduction of the viral load under the limit of detection (250 copies/mL) after 24 weeks of treatment completion.
Results: Sustained control of HBV DNA was significantly more common in NASVAC group (p<0.05) at 24 weeks of follow up. NASVAC-induced increases of alanine aminotransferases (ALT) were detected in 85% patients after 5 nasal vaccinations, although seen in only 30% of patients receiving Peg-IFN. At the end of treatment (EOT) antiviral effect was comparable in both NASVAC and Peg-IFN groups. Clearance of Hepatitis B e antigen (HBeAg) was also more frequent in NASVAC group compared to Peg-IFN recipients. A lower progression to cirrhosis was found in NASVAC group compared to Peg-IFN group.
Conclusion: Nasvac induced a superior reduction of the viral load under the limit of detection compared to Peg-IFN treatment. It is a safe and efficacious finite alternative of antiviral treatment for CHB patients.
Trial registration: ClinicalTrials.gov NCT 01374308.
Trial registration: ClinicalTrials.gov NCT01374308.
Conflict of interest statement
This study was partly funded by the Clinical Research Organization Bangladesh. There are no patents, products in development or marketed products to declare. This does not alter our adherence to all the PLOS ONE policies on sharing data and materials, as detailed online in the guide for authors.
Figures


References
-
- Lavanchy D. Hepatitis B virus epidemiology, disease burden, treatment, and current and emerging prevention and control measures. J Viral Hepat 2004;11:97–107. - PubMed
-
- World Health Organization. Hepatitis B. Fact sheet No. 204. Available at: http://www.who.int/mediacentre/factsheets/fs204/ (accessed April 12th 2017)
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

