Intravitreal pro-inflammatory cytokines in non-obese diabetic mice: Modelling signs of diabetic retinopathy
- PMID: 30133488
- PMCID: PMC6105000
- DOI: 10.1371/journal.pone.0202156
Intravitreal pro-inflammatory cytokines in non-obese diabetic mice: Modelling signs of diabetic retinopathy
Abstract
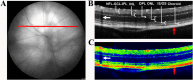
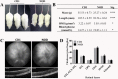
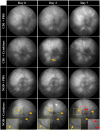
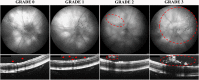
Diabetic retinopathy is a vascular disease of the retina characterised by hyperglycaemic and inflammatory processes. Most animal models of diabetic retinopathy are hyperglycaemia-only models that do not account for the significant role that inflammation plays in the development of the disease. In the present study, we present data on the establishment of a new animal model of diabetic retinopathy that incorporates both hyperglycaemia and inflammation. We hypothesized that inflammation may trigger and worsen the development of diabetic retinopathy in a hyperglycaemic environment. Pro-inflammatory cytokines, IL-1β and TNF-α, were therefore injected into the vitreous of non-obese diabetic (NOD) mice. CD1 mice were used as same genetic background controls. Fundus and optical coherence tomography images were obtained before (day 0) as well as on days 2 and 7 after intravitreal cytokine injection to assess vessel dilation and beading, retinal and vitreous hyper-reflective foci and retinal thickness. Astrogliosis and microgliosis were assessed using immunohistochemistry. Results showed that intravitreal cytokines induced vessel dilation, beading, severe vitreous hyper-reflective foci, retinal oedema, increased astrogliosis and microglia upregulation in diabetic NOD mice. Intravitreal injection of inflammatory cytokines into the eyes of diabetic mice therefore appears to provide a new model of diabetic retinopathy that could be used for the study of disease progression and treatment strategies.
Conflict of interest statement
We, the authors declare that no competing interests exist.
Figures
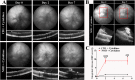
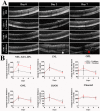
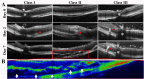
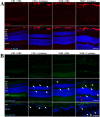
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

