Enhanced accumulation of harpagide and 8-O-acetyl-harpagide in Melittis melissophyllum L. agitated shoot cultures analyzed by UPLC-MS/MS
- PMID: 30133513
- PMCID: PMC6104996
- DOI: 10.1371/journal.pone.0202556
Enhanced accumulation of harpagide and 8-O-acetyl-harpagide in Melittis melissophyllum L. agitated shoot cultures analyzed by UPLC-MS/MS
Abstract
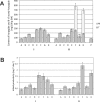
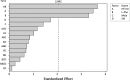
Harpagide and its derivatives have valuable medicinal properties, such as anti-inflammatory, analgesic and potential antirheumatic effects. There is the demand for searching plant species containing these iridoids or developing biotechnological methods to obtain the compounds. The present study investigated the effects of methyl jasmonate (MeJa, 50 μM), ethephon (Eth, 50 μM) and L-phenylalanine (L-Phe, 2.4 g/L of medium), added to previously selected variant of Murashige and Skoog medium (supplemented with plant growth regulators: 6-benzylaminopurine 1.0 mg/L, α-naphthaleneacetic acid 0.5 mg/L, gibberellic acid 0.25 mg/L) on the accumulation of harpagide and 8-O-acetyl-harpagide in Melittis melissophyllum L. agitated shoot cultures. Plant material was harvested 2 and 8 days after the supplementation. Iridoids were quantitatively analyzed by the UPLC-MS/MS method in extracts from the biomass and the culture medium. It was found that all of the variants caused an increase in the accumulation of harpagide. In the biomass harvested after 2 days, the highest harpagide content of 247.3 mg/100 g DW was found for variant F (L-Phe and Eth), and the highest 8-O-acetyl-harpagide content of 138 mg/100 g DW for variant E (L-Phe and MeJa). After 8 days, in some variants, a portion of the metabolites was released into the culture medium. Considering the total amount of the compounds (in the biomass and medium), the highest accumulation of harpagide, amounting to 619 mg/100 g DW, was found in variant F, and the highest amount of 8-O-acetyl-harpagide, of 255.4 mg/100 g DW, was found in variant H (L-Phe, MeJa, Eth) when harvested on the 8th day. These amounts were, respectively, 24.7 and 4.8 times higher than in the control culture, and were, respectively, 15 and 6.7 times higher than in the leaves of the soil-grown plant. The total amount of the two iridoids was highest for variant F (0.78% DW) and variant H (0.68% DW) when harvested on the 8th day. The results indicate that the agitated shoot cultures of M. melissophyllum can be a rich source of harpagide and 8-O-acetyl-harpagide, having a potential practical application. To the best of our knowledge we present for the first time the results of the quantitative UPLC-MS/MS analysis of harpagide and 8-O-acetyl-harpagide in M. melissophyllum shoot cultures and the enhancement of their accumulation by means of medium supplementation with elicitors and precursor.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures





















References
-
- Harpagophyti radix. In European Pharmacopoeia 9thEdition.: European Directorate for the Quality of Medicines, Council of Europe; 2017. p. 1335.
-
- Smetanska I. Production of Secondary Metabolites Using Plant Cell Cultures In: Stahl U, Donalies U, Nevoigt E, editors. Food Biotechnology. Berlin-Heidelberg: Springer; 2008. p. 187–228. - PubMed
-
- Karuppusamy S. A review on trends in production of secondary metabolites from higher plants by in vitro tissue, organ and cell cultures. J Med Plants Res. 2009; 3(13): 1222–39.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

