IL-13-regulated Macrophage Polarization during Granuloma Formation in an In Vitro Human Sarcoidosis Model
- PMID: 30134122
- PMCID: PMC6348723
- DOI: 10.1165/rcmb.2018-0053OC
IL-13-regulated Macrophage Polarization during Granuloma Formation in an In Vitro Human Sarcoidosis Model
Abstract
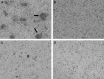
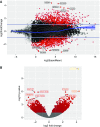
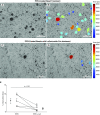
The mechanisms underlying abnormal granuloma formation in patients with sarcoidosis are complex and remain poorly understood. A novel in vitro human granuloma model was used to determine the molecular mechanisms of granuloma genesis in patients with sarcoidosis in response to putative disease-causing mycobacterial antigens. Peripheral blood mononuclear cells (PBMCs) from patients with active sarcoidosis and from normal, disease-free control subjects were incubated for 7 days with purified protein derivative-coated polystyrene beads. Molecular responses, as reflected by differential expression of genes, extracellular cytokine patterns, and cell surface receptor expression, were analyzed. Unbiased systems biology approaches were used to identify signaling pathways engaged during granuloma formation. Model findings were compared with human lung and mediastinal lymph node gene expression profiles. Compared with identically treated PBMCs of control subjects (n = 5), purified protein derivative-treated sarcoidosis PBMCs (n = 6) were distinguished by the formation of cellular aggregates resembling granulomas. Ingenuity Pathway Analysis of differential expression gene patterns identified molecular pathways that are primarily regulated by IL-13, which promotes alternatively activated (M2) macrophage polarization. M2 polarization was further demonstrated by immunohistochemistry performed on the in vitro sarcoidosis granuloma-like structures. IL-13-regulated gene pathways were confirmed in human sarcoidosis lung and mediastinal lymph node tissues. The in vitro human sarcoidosis granuloma model provides novel insights into early granuloma formation, particularly IL-13 regulation of molecular networks that regulate M2 macrophage polarization. M2 macrophages are predisposed to aggregation and multinucleated giant cell formation, which are characteristic features of sarcoidosis granulomas. Clinical trial registered with www.clinicaltrials.gov (NCT01857401).
Keywords: AmpliSeq; macrophage; peripheral blood mononuclear cells; purified protein derivative.
Figures


Comment in
-
Macrophage Polarization in Sarcoidosis: An Unexpected Accomplice?Am J Respir Cell Mol Biol. 2019 Jan;60(1):9-10. doi: 10.1165/rcmb.2018-0298ED. Am J Respir Cell Mol Biol. 2019. PMID: 30281325 Free PMC article. No abstract available.
References
-
- Grunewald J, Spagnolo P, Wahlström J, Eklund A. Immunogenetics of disease-causing inflammation in sarcoidosis. Clin Rev Allergy Immunol. 2015;49:19–35. - PubMed
-
- Helming L, Gordon S. Macrophage fusion induced by IL-4 alternative activation is a multistage process involving multiple target molecules. Eur J Immunol. 2007;37:33–42. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

