Living Neurons with Tau Filaments Aberrantly Expose Phosphatidylserine and Are Phagocytosed by Microglia
- PMID: 30134156
- PMCID: PMC6161320
- DOI: 10.1016/j.celrep.2018.07.072
Living Neurons with Tau Filaments Aberrantly Expose Phosphatidylserine and Are Phagocytosed by Microglia
Abstract
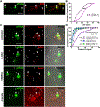
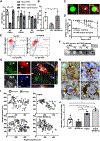
Tau protein forms insoluble filamentous inclusions that are closely associated with nerve cell death in many neurodegenerative diseases. How neurons die in these tauopathies is unclear. We report that living neurons with tau inclusions from P301S-tau mice expose abnormally high amounts of phosphatidylserine because of the production of reactive oxygen species (ROS). Consequently, co-cultured phagocytes (BV2 cells or primary microglia) identify and phagocytose the living neurons, thereby engulfing insoluble tau inclusions. To facilitate engulfment, neurons induce contacting microglia to secrete the opsonin milk-fat-globule EGF-factor-8 (MFGE8) and nitric oxide (NO), whereas neurons with tau inclusions are rescued when MFGE8 or NO production is prevented. MFGE8 expression is elevated in transgenic P301S-tau mouse brains with tau inclusions and in tau inclusion-rich brain regions of several human tauopathies, indicating shared mechanisms of disease. Preventing phagocytosis of living neurons will preserve them for treatments that inhibit tau aggregation and toxicity.
Keywords: MFGE8; cell death; microglia; neurodegeneration; neuroinflammation; nitric oxide; phagocytosis; phosphatidylserine; tau; tauopathies.
Copyright © 2018 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
DECLARATION OF INTERESTS
The authors declare no competing interests.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

