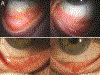
Brinzolamide-induced Follicular Conjunctivitis
- PMID: 30134369
- PMCID: PMC6218271
- DOI: 10.1097/IJG.0000000000001063
Brinzolamide-induced Follicular Conjunctivitis
Abstract
We report a case of a patient who developed a severe case of follicular conjunctivitis from brinzolamide after 1.5 years of consistent use. This patient was rechallenged again after resolution of the follicles and subsequently redeveloped similar conjunctivitis. This is the first confirmed reported case of follicular conjunctivitis from brinzolamide use.
Figures
References
-
- Katz LJ. Twelve-month evaluation of brimonidine-purite versus brimonidine in patients with glaucoma or ocular hypertension. J Glaucoma 2002;11(2):119–26. - PubMed
-
- Fraunfelder FT, Fraunfelder FW, Chambers WA. Clinical Ocular Toxicology E-Book:Drug-Induced Ocular Side Effects: Elsevier Health Sciences, 2008.
-
- Silver LH. Clinical efficacy and safety of brinzolamide (Azopt), a new topical carbonic anhydrase inhibitor for primary open-angle glaucoma and ocular hypertension. Brinzolamide Primary Therapy Study Group. Am J Ophthalmol 1998;126(3):400–8. - PubMed
-
- March WF, Ochsner KI. The long-term safety and efficacy of brinzolamide 1.0% (azopt) in patients with primary open-angle glaucoma or ocular hypertension. The Brinzolamide Long-Term Therapy Study Group. Am J Ophthalmol 2000;129(2):136–43. - PubMed
-
- Manni G, Centofanti M, Sacchetti M, et al. Demographic and clinical factors associated with development of brimonidine tartrate 0.2%-induced ocular allergy. J Glaucoma 2004;13(2):163–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical